Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad Reza Boorboori | + 2296 word(s) | 2296 | 2021-09-09 05:39:28 | | | |
| 2 | Rita Xu | Meta information modification | 2296 | 2021-09-16 12:03:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Boorboori, M.R. Arsenic Concentration/Toxicity in Rice. Encyclopedia. Available online: https://encyclopedia.pub/entry/14254 (accessed on 07 February 2026).
Boorboori MR. Arsenic Concentration/Toxicity in Rice. Encyclopedia. Available at: https://encyclopedia.pub/entry/14254. Accessed February 07, 2026.
Boorboori, Mohammad Reza. "Arsenic Concentration/Toxicity in Rice" Encyclopedia, https://encyclopedia.pub/entry/14254 (accessed February 07, 2026).
Boorboori, M.R. (2021, September 16). Arsenic Concentration/Toxicity in Rice. In Encyclopedia. https://encyclopedia.pub/entry/14254
Boorboori, Mohammad Reza. "Arsenic Concentration/Toxicity in Rice." Encyclopedia. Web. 16 September, 2021.
Copy Citation
Rice is one of the most important routes for arsenic to enter the human food chain and threatens more than half of the world’s population. In addition, arsenic-contaminated soils and waters increase the concentration of this element in various tissues of rice plants.
rice
arsenic
silicon
phosphorus
selenium
calcium
1. Introduction
Agriculture is currently facing the challenge of providing adequate food for a growing population [1]. Rice is a staple food worldwide [2], and it provides two-thirds of the calories needed for two billion people in Asia; while also being the primary protein source for those people [3][4]. Different types of rice provide essential elements, vitamins, nutrients, and fibre for the human body [5]. However, rice is one of the most widely consumed plants globally and requires a constant water source through irrigation, and plants consume about 60% of the irrigation water. Soil texture affects the uptake and growth of plant nutrients because it alters access to water in the soil [1][6][7]. One of the most important threats to natural and human ecosystems is the pollution of water and soil resources by heavy metals and metals, which causes fundamental changes in ecosystems while their entry into the biological cycle can have devastating environmental effects [8][9]. Recently, contamination of soils and groundwater with heavy metals has become a serious problem. They harm crop production worldwide [10][11], and also, contaminated soil harms economic growth and development due to the negative effects on agricultural products [12][13].
2. Arsenic (As) and Rice
Arsenic is a carcinogenic metalloid, and humans are contaminated mainly through diet and drinking water., increasing health problems [14]. The National Toxicology Program and International Agency for Research on Cancer categorized arsenic as the first carcinogenic agent [15][16]. There are various species of As in nature, and they are listed in Table 1. More than other grains, rice accumulates arsenic in its tissues [17]. The kinds of As in rice are mainly inorganic (iAs, including arsenate and arsenite), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) [18]. According to research, inorganic kinds of As are more toxic than DMA and MMA [19], and under anaerobic situations, As (III) is the prevailing type in soil solutions [20].
Table 1. The list of various species of arsenic.
| Arsenic Compounds | Acronyms | Chemical Formula |
|---|---|---|
| Arsenate | As (V) | As(O−)3 |
| Arsenite | As (III) | O=As(O−)3 |
| Methylarsonate | MMA | CH3AsO(O−)2 |
| Dimethylarsinate | DMA | (CH3)2AsO(O−) |
| Trimethylarsin oxide | TMAO | (CH3)3AsO |
| Tetramethylarsonium ion | TETRA | (CH3)4As+ |
| Arsenobetain | AB | (CH3)3As+CH2COO− |
| Trimethylarsoniopropionate | TMAP | (CH3)3As+CH2CH2COO− |
| Arsenocholine | AC | (CH3)3As+CH2CH2O− |
| Dimethylarsinoylacetate | DMAA | (CH3)2(O)As+CH2COO− |
| Dimethylarsinoylpropionate | DMAP | (CH3)2(O)As+CH2CH2COO− |
Nomenclature is as proposed by Francesconi and Kuehnelt [21].
Increased arsenic release from soil particles by rice occurs through competition with soil mineral adsorption sites [22]. In anaerobic environments, arsenic motility and viability are significantly increased [20]. Paddy fields are an excellent environment for increasing arsenic bioavailability—the amount of arsenic absorption is such that it can enter the rice and affect rice growth—because the rice plants are grown in flooded conditions [23]. Therefore, since arsenic has a high solubility in water, this element is the most critical pollutant [24]. Arsenic contamination has become the reason for significant health difficulties in humans and animals [25], and in India, China, Sri Lanka, Pakistan, Nepal, Bangladesh, Chile, Mexico, and Argentina, pollution of drinking water sources with arsenic is now a public health emergency [26]. In some mines in southern China’s Guangdong, the soil is heavily polluted with arsenic and some other heavy metals, and according to some studies, pollution in agricultural lands around these mines has reached 344.11 mg/kg, which indicates an excessive concentration of arsenic (1 to 40 mg/kg) in the soil [27].
There are two main ways to absorb arsenic in rice: (1) As (V) as a chemical analogue of phosphate is absorbed in rice roots by the phosphate transport protein system. (2) As (III) is an analogue of silicic acid and can be absorbed via the roots of rice with the delivery systems of silicic acid (Lsi1 and Lsi2) (Figure 1) [28][29]. As mentioned, there are two main pathways for arsenic uptake by rice, so because we wanted to investigate the role of microelements and macroelements in reducing toxicity and uptake of arsenic, we looked at elements that have a common uptake pathway with arsenic. Among them, we examined two macroelements (P, Ca) and two microelements (Si, Se) that effectively reduce the toxicity and absorption of arsenic in rice.
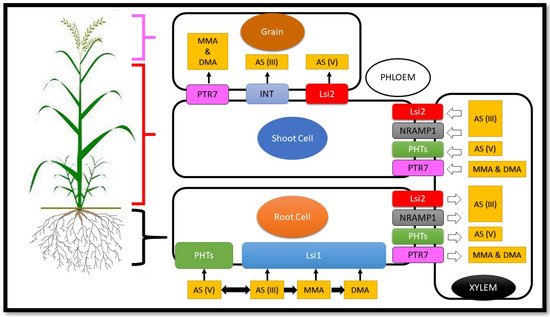
Figure 1. Mechanisms of arsenic uptake, transport, accumulation, and distribution in rice. Nomenclature is as proposed by Shinde and Kumar [30] with some modifications.
3. Silicon (Si) and As
Si is an essential element in the soil and crust of the earth, and soil Si content makes up 1 to 45% of soil’s dry weight, but only 0.1 to 0.6% is soluble [33][34]. Si is a motionless element in plants, and rice is absorbed as ionized Si(OH)3O and silicic acid (H4SiO4). Si content of rice’s dry weight can reach up to 10% [35][36], and rice is a stacked Si plant, often used as a sample plant for Si uptake, transport, and accumulation [3]. According to research, silicon is a practical and valuable element for plant growth, especially in various stress conditions such as plant diseases, plant pests, drought, salinity [37], while one of the significant effects of Si is in reducing the toxicity of metal by decreasing absorption and transport of metals in plants. [38].
Studies have shown that rice roots absorb arsenite via two Si carriers, Lsi1 (the aquaporin NIP2; 1) and Lsi2 (an efflux carrier) [39]. Lsi1 is an invasive transporter responsible for transporting silicon and arsenic (III) from culture solutions to rice root cells [40]. As (III) is absorbed within cells in Escherichia coli, the yeast (Saccharomyces cerevisiae) via aquaglyceroporins and several aquaporin channels of plants are dependent on the Nodulin 26 subfamily, the same as the intrinsic protein (NIP) is permeable to As (III) in the case of heterogeneity in yeast [41][42]. The Si rice carrier Lsi1 (OsNIP2; 1) is also penetrable to As (III) expressed in Xenopus laevis and yeast oocytes [39]. The second silicon transporter (Lsi2) in rice roots transfers silicon efflux from epidermal and endodermal cells to stele to load xylem, and this transporter also mediates the efflux of As (III) [39][43].
However, adding Si to soil and water significantly decreases the concentration of arsenic in rice. In addition, some studies have shown that Si can help increase methylation in rice tissue and affect the concentration of different As types in rice [44]. Therefore, adding silicon to the soil can reduce As uptake and accumulation in rice, and Si foliar application may also be an alternative route to reduce arsenic accumulation in rice [22].
Different studies have been performed on the efficacy of Si in reducing the uptake and transport of arsenic in rice. For instance, Seyfferth and Fendorf [44] detected that the concentration of As in the grain was significantly reduced by adding Si to the pore water. Si application has also been reported to decrease concentrations of different types of arsenic in stem, leaf, husk, and brown rice (Figure 2) [45].
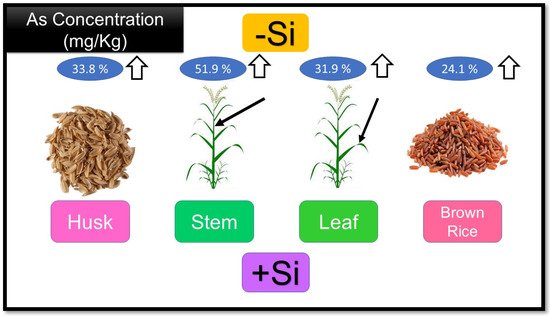
Figure 2. The effect of Adding silicon (+Si) and without silicon (−Si) treatments on arsenic concentration on different rice tissue. Nomenclature is as proposed by Gang, ZHENG [46].
It has also been observed that Si usage significantly decreases the concentration of iAs, while the DMA concentration increases in rice plant tissues [45]. It was found that silicic acid can also dislocate arsenite, arsenate, and Dimethylarsinate from the surface of soil particles, which increases the aggregate concentration of arsenic in the soil solution [47]. Hence, the effects of adding silicon to soil on arsenic bioavailability depend on the interactions between As and Si in pore water, particles of soil, and the absorption of rice roots [48]. According to research, to control the adverse efficacy of silicon usage in paddy soil, Si foliar usage can be a more impressive way to affect the arsenic accumulation rate in rice [22].
4. Phosphorus (P) and As
Phosphorus is a vital element required for growing plants, and plants absorb it mainly as inorganic phosphate (Pi) [49]. Phosphorus plays an essential role in the biochemistry and physiology of plants, and almost all phospholipids, nucleic acids, and ATPs contain P, which is involved in regulating significant metabolic pathways and enzymatic reactions, making phosphorus essential for the worldwide production of sustainable food [50][51]. Agricultural products consume 90% of the extracted non-renewable phosphate reserves annually. As the population of the world increases and phosphate resources are depleted, it is necessary to improve the phosphorus use efficiency (PUE) at the plant and farm-wide scale [50][52]. Due to the high stabilization of phosphorus in the soil and its slow release to the root surface, plants have different strategies to enable phosphorus accessibility from the ground [53].
Arsenic and phosphorus are chemical analogues. However, some of the chemical properties of P and As, including redox reactions, are distinct and cause the biochemical behaviour of the two elements to be different. As mentioned, P is an essential plant growth element, while arsenic is toxic and prevents plant growth [54][55]. In the environment, arsenic occurs as arsenate or arsenite (e.g., AsO4−3 or AsO3−3); in contrast, phosphorus exists mainly as orthophosphate ions (PO4−3). Phosphates usually have many physicochemical properties similar to arsenates, like symmetry, acid decomposition constants, and ion size, but P is more susceptible to transport sites than As (V) [55][56][57]. In aqueous conditions, As (V) and P (V) are present as arsenates (AsO4−3) and phosphate (PO4−3), which are surrounded by four oxygen atoms in quadrilateral coordination. Arsenate and phosphate are assumed as chemical analogues due to their being similar chemical species, which can replace each other in chemical reactions. Biogeochemical reactions often are seen as precipitation/dissolution, adsorption/excretion, and competitive/adsorption reactions in plant and microbial systems [58][59][60][61].
Competitive processes of biogeochemical reaction between arsenate and phosphate control the arsenic bioavailability in the environment. However, arsenic oxidation reactions in the atmosphere produce various biogeochemical responses which do not occur for P. For instance, in anaerobic conditions like rice paddies, arsenate is transformed to arsenite because it is not absorbed as much as arsenate on soil particles (especially at low or neutral pH), so, it is more mobile and soluble. Redox mobility creates arsenic distribution in sediments and soils that decreases under fluctuations of oxidation, but P is not changed by redox variation [62]. An experiment on disposal of arsenate showed after using phosphate solutions, only 35% of the adsorbed arsenate was absorbed from goethite, which indicates that the arsenate bonds were more substantial than the phosphate bonds on iron oxide. The toxicity mechanism in organisms is maybe metabolic substitution of arsenate for phosphate [63].
Under aerobic conditions, As (V) and phosphorus (Pi) due to similar chemical properties, stable quadrilateral oxidants form the oxidation state þ5. However, there is less correlation between the behaviour of phosphate and arsenite under aqueous conditions because phosphate is not an analogue of As (III), and arsenite transport is not affected by phosphate [64][65]. However, arsenite oxidation to arsenate is distinct in the rice root region [66]. Thus, the effect of phosphate on the behaviour of arsenite in the rhizosphere cannot be completely ignored, and actual cognition of this feature is needed. Unlike hydroponics, P usage in the soil increases arsenic uptake by the plants because of arsenic excretion [67].
Due to the essential role of phosphate in controlling the solubility of arsenic in the soil and its uptake through plants, special attention to P in paddy fields should be given [68]; in addition, the simultaneous usage of calcium and phosphorus forms the Ca-P-As complex and reduces mobility in enriched soils, [69]. Because As and P interactions in the soil-plant system are complicated, most studies have failed, while the variability competition between P and As depends on substrate conditions and soil type [70][71][72].
The much higher application of P may help compete for arsenic uptake in plants. In contrast, increasing the concentration of arsenic with increasing P in the soil solution may also increase arsenic toxicity because of As excretion in the soil solution. On the other hand, excessive P usage for the plants may also enhance the environmental risks [73]. For example, triple superphosphate (TSP) is vital among the phosphorus fertilizers, and the raw material of TSP, phosphate rock, may contain arsenic and release arsenic from solid soils into the soil solution [65].
At similar concentrations of P and As, arsenic is more available for uptake by the plants because of the smaller size of arsenic, and the charge of P ions binds to the soil with a higher intensity than As (V) [64][74]. In addition, P competes with As (V) over time because of the lower soil uptake of P, and according to the stock charge hypothesis, ligand exchange theory, and Steindorf–Rehbon–Shintoch equation, Pi is more likely to be replaced by As (V) in soil [64][75].
Soil properties, soil texture, soil mineralogy, and environmental factors may have a significant impact on soil availability, mobility, and interaction of As-Pi [76], and uptake through plants, including mineral components, the presence of anions (e.g., microbial activity, organic matter (OM), redox potential, citrate, phosphate, pH, phytates/phytic acid, and in particular, iron oxide and Al oxide/hydroxides) [77][78]. Amid these factors, pH and goethite firmly control the behaviour of P and As. Iron and magnesium-rich minerals such as phronesis smectites, nontronite, goethite, bronchitis, and pyrolusite absorb more As (V) than Pi when prepared in molar ratio. However, Pi absorbs more than As (V) in non-crystalline allergens containing allophane, boehmite, gibbsite, and clay sections (e.g., vermiculite, illite, and kaolinite) [79][80]. Goethite’s high levels in the soil can decrease P and As uptake by plants. Adding Fe and P to high arsenic soils using a sequential combination method can reduce arsenic toxicity to plant roots by providing a food source [81][82].
References
- Alhaj Hamoud, Y.; Wang, Z.; Guo, X.; Shaghaleh, H.; Sheteiwy, M.; Chen, S.; Qiu, R.; Elbashier, M. Effect of irrigation regimes and soil texture on the potassium utilization efficiency of rice. Agronomy 2019, 9, 100.
- Liang, Y.; Nan, W.; Qin, X.; Zhang, H. Field performance on grain yield and quality and genetic diversity of overwintering cultivated rice (Oryza sativa L.) in southwest China. Sci. Rep. 2021, 11, 1–16.
- Kim, Y.-H.; Khan, A.L.; Shinwari, Z.K.; Kim, D.-H.; Waqas, M.; Kamran, M.; Lee, I.-J. Silicon treatment to rice (Oryza sativa L. cv.‘Gopumbyeo’) plants during different growth periods and its effects on growth and grain yield. J. Pak. J. Bot. 2012, 44, 891–897.
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261.
- Maione, C.; Barbosa, R.M. Recent applications of multivariate data analysis methods in the authentication of rice and the most analyzed parameters: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1868–1879.
- Adarsh, S.; Thomas, G. Artificial groundwater recharge through rice (Oryza sativa L.) cultivation: A systematic review. Int. J. Chem. Stud. 2019, 7, 1856–1860.
- Arunrat, N.; Pumijumnong, N.; Sereenonchai, S.; Chareonwong, U.; Wang, C. Assessment of climate change impact on rice yield and water footprint of large-scale and individual farming in Thailand. Sci. Total Environ. 2020, 726, 137864.
- Bhattacharya, P.; Samal, A.; Majumdar, J.; Santra, S. Accumulation of arsenic and its distribution in rice plant (Oryza sativa L.) in Gangetic West Bengal, India. J. Paddy Water Environ. 2010, 8, 63–70.
- Bhattacharya, S.; Gupta, K.; Debnath, S.; Ghosh, U.C.; Chattopadhyay, D.; Mukhopadhyay, A. Arsenic bioaccumulation in rice and edible plants and subsequent transmission through food chain in Bengal basin: A review of the perspectives for environmental health. J. Toxicol. Environ. Chem. 2012, 94, 429–441.
- Raza, M.M.; Ullah, S.; Ahmad, Z.; Saqib, S.; Ahmad, S.; Bilal, H.M.; Wali, F. Silicon mediated arsenic reduction in rice by limiting its uptake. J. Agric. Sci. China 2016, 7, 1.
- Dey, T.K.; Banerjee, P.; Bakshi, M.; Kar, A. Groundwater arsenic contamination in West Bengal: Current scenario, effects and probable ways of mitigation. J. Int. Lett. Nat. Sci. 2014, 8, 45–58.
- Farquhar, G.D.; Buckley, T.N.; Miller, J.M. Optimal stomatal control in relation to leaf area and nitrogen content. J. Silva Fennica. 2002, 36, 625–637.
- Huq, S.I.; Joardar, J.; Parvin, S.; Correll, R.; Naidu, R. Arsenic contamination in food-chain: Transfer of arsenic into food materials through groundwater irrigation. J. Health Popul. 2006, 24, 305.
- Cui, J.; Shi, J.; Jiang, G.; Jing, C. Arsenic levels and speciation from ingestion exposures to biomarkers in Shanxi, China: Implications for human health. Environ. Sci. Technol. 2013, 47, 5419–5424.
- Jørgensen, K.; Larsen, E.H.; Petersen, A.; Lund, K.H.; Hilbert, G.; Andersen, N.L.; Hallas-Møller, T.; Larsen, J.C. Chemical contaminants. Food Monit. 1993, 1997, 133.
- Bahler, D.; Stone, B.; Wellington, C.; Bristol, D.W. Symbolic, neural, and Bayesian machine learning models for predicting carcinogenicity of chemical compounds. J. Chem. Inf. Comput. Sci. 2000, 40, 906–914.
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007, 41, 6854–6859.
- Nookabkaew, S.; Rangkadilok, N.; Mahidol, C.; Promsuk, G.; Satayavivad, J. Determination of arsenic species in rice from Thailand and other Asian countries using simple extraction and HPLC-ICP-MS analysis. J. Agric. Food Chem. 2013, 61, 6991–6998.
- Calatayud, M.; Bralatei, E.; Feldmann, J.; Devesa, V.; Vélez, D. Transformation of arsenic species during in vitro gastrointestinal digestion of vegetables. J. Agric. Food Chem. 2013, 61, 12164–12170.
- Xu, X.; McGrath, S.; Meharg, A.; Zhao, F. Growing rice aerobically markedly decreases arsenic accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579.
- Francesconi, K.A.; Kuehnelt, D. Determination of arsenic species: A critical review of methods and applications, 2000–2003. Analyst 2004, 129, 373–395.
- Zhang, S.; Geng, L.; Fan, L.; Zhang, M.; Zhao, Q.; Xue, P.; Liu, W. Spraying silicon to decrease inorganic arsenic accumulation in rice grain from arsenic-contaminated paddy soil. Sci. Total Environ. 2020, 704, 135239.
- Pan, W.; Wu, C.; Xue, S.; Hartley, W. Arsenic dynamics in the rhizosphere and its sequestration on rice roots as affected by root oxidation. J. Environ. Sci. 2014, 26, 892–899.
- Pinto, A.; Mota, A.D.; De Varennes, A.; Pinto, F. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. J. Sci. Total Environ. 2004, 326, 239–247.
- Chowdhury, T.R.; Basu, G.K.; Mandal, B.K.; Biswas, B.K.; Samanta, G.; Chowdhury, U.K.; Chanda, C.R.; Lodh, D.; Roy, S.L.; Saha, K.C. Arsenic poisoning in the Ganges delta. J. Nat. 1999, 401, 545–546.
- Petrusevski, B.; Sharma, S.; Schippers, J.C.; Shordt, K. Arsenic in drinking water. J. Delft IRC Int. Water Sanit. Cent. 2007, 17, 36–44.
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235.
- Wu, C.; Zou, Q.; Xue, S.; Mo, J.; Pan, W.; Lou, L.; Wong, M.H. Effects of silicon (Si) on arsenic (As) accumulation and speciation in rice (Oryza sativa L.) genotypes with different radial oxygen loss (ROL). J. Chemosphere 2015, 138, 447–453.
- Zhao, F.-J.; Zhu, Y.-G.; Meharg, A.A. Methylated arsenic species in rice: Geographical variation, origin, and uptake mechanisms. J. Environ. Sci. Technol. 2013, 47, 3957–3966.
- Shinde, A.; Kumar, K. Mechanisms of Arsenic Transport, Accumulation, and Distribution in Rice. In Arsenic Toxicity: Challenges and Solutions; Springer: Berlin/Heidelberg, Germany, 2021; p. 279.
- He, Y.; Pedigo, C.E.; Lam, B.; Cheng, Z.; Zheng, Y. Bioaccessibility of arsenic in various types of rice in an in vitro gastrointestinal fluid system. J. Environ. Sci. Health Part B 2012, 47, 74–80.
- Meharg, A.A.; Jardine, L. Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol. 2003, 157, 39–44.
- Cuong, T.X.; Ullah, H.; Datta, A.; Hanh, T.C. Effects of silicon-based fertilizer on growth, yield and nutrient uptake of rice in tropical zone of Vietnam. J. Rice Sci. 2017, 24, 283–290.
- Song, Z.; Wang, H.; Strong, P.J.; Shan, S. Increase of available soil silicon by Si-rich manure for sustainable rice production. J. Agron. Sustain. Dev. 2014, 34, 813–819.
- Ma, J.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. Stud. Plant Sci. 2001, 8, 17–39.
- Pontigo, S.; Godoy, K.; Jiménez, H.; Gutiérrez-Moraga, A.; Mora, M.D.L.L.; Cartes, P. Silicon-mediated alleviation of aluminum toxicity by modulation of Al/Si uptake and antioxidant performance in ryegrass plants. J. Front. Plant Sci. 2017, 8, 642.
- Liang, Y.; Wong, J.; Wei, L. Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 2005, 58, 475–483.
- Fleck, A.T.; Mattusch, J.; Schenk, M.K. Silicon decreases the arsenic level in rice grain by limiting arsenite transport. J. Plant Nutr. 2013, 176, 785–794.
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.-Y.; Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935.
- Li, R.-Y.; Ago, Y.; Liu, W.-J.; Mitani, N.; Feldmann, J.; McGrath, S.P.; Ma, J.F.; Zhao, F.-J. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009, 150, 2071–2080.
- Bhattacharjee, H.; Rosen, B.P. Arsenic metabolism in prokaryotic and eukaryotic microbes. In Molecular Microbiology of Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2007; pp. 371–406.
- Bhattacharjee, H.; Mukhopadhyay, R.; Thiyagarajan, S.; Rosen, B.P. Aquaglyceroporins: Ancient channels for metalloids. J. Biol. 2008, 7, 1–6.
- Ma, J.F.; Yamaji, N.; Tamai, K.; Mitani, N. Genotypic difference in silicon uptake and expression of silicon transporter genes in rice. J. Plant Physiol. 2007, 145, 919–924.
- Seyfferth, A.L.; Fendorf, S. Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). J. Environ. Sci. Technol. 2012, 46, 13176–13183.
- Liu, W.-J.; McGrath, S.P.; Zhao, F.-J. Silicon has opposite effects on the accumulation of inorganic and methylated arsenic species in rice. J. Plant Soil 2014, 376, 423–431.
- Gang, L.; Zheng, M.; Tang, J.; Hojae, S.; Chao, C. Effect of silicon on arsenic concentration and speciation in different rice tissues. Pedosphere 2018, 28, 511–520.
- Lee, C.-H.; Huang, H.-H.; Syu, C.-H.; Lin, T.-H.; Lee, D.-Y. Increase of As release and phytotoxicity to rice seedlings in As-contaminated paddy soils by Si fertilizer application. J. Hazard. Mater. 2014, 276, 253–261.
- Syu, C.-H.; Huang, C.-C.; Jiang, P.-Y.; Chien, P.-H.; Wang, H.-Y.; Su, J.-Y.; Lee, D.-Y. Effects of foliar and soil application of sodium silicate on arsenic toxicity and accumulation in rice (Oryza sativa L.) seedlings grown in As-contaminated paddy soils. Soil Sci. Plant Nutr. 2016, 62, 357–366.
- Cao, Y.; Sun, D.; Chen, J.-X.; Mei, H.; Ai, H.; Xu, G.; Chen, Y.; Ma, L.Q. Phosphate transporter PvPht1; 2 enhances phosphorus accumulation and plant growth without impacting arsenic uptake in plants. Environ. Sci. Technol. 2018, 52, 3975–3981.
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305.
- Rose, T.J.; Impa, S.; Rose, M.; Pariasca-Tanaka, J.; Mori, A.; Heuer, S.; Johnson-Beebout, S.; Wissuwa, M. Enhancing phosphorus and zinc acquisition efficiency in rice: A critical review of root traits and their potential utility in rice breeding. Ann. Bot. 2013, 112, 331–345.
- Simpson, R.J.; Oberson, A.; Culvenor, R.A.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A. Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant Soil 2011, 349, 89–120.
- Wu, P.; Shou, H.; Xu, G.; Lian, X. Improvement of phosphorus efficiency in rice on the basis of understanding phosphate signaling and homeostasis. Curr. Opin. Plant Biol. 2013, 16, 205–212.
- Farooq, M.A.; Islam, F.; Ali, B.; Najeeb, U.; Mao, B.; Gill, R.A.; Yan, G.; Siddique, K.H.; Zhou, W. Arsenic toxicity in plants: Cellular and molecular mechanisms of its transport and metabolism. Environ. Exp. Bot. 2016, 132, 42–52.
- Strawn, D.G. Review of interactions between phosphorus and arsenic in soils from four case studies. Geochem. Trans. 2018, 19, 1–13.
- Beever, R.E.; Burns, D. Phosphorus uptake, storage and utilization by fungi. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 1981; Volume 8, pp. 127–219.
- Dunlop, J.; Phung, H.; Meeking, R.; White, D. The kinetics associated with phosphate absorption by Arabidopsis and its regulation by phosphorus status. Funct. Plant Biol. 1997, 24, 623–629.
- Neupane, G.; Donahoe, R.J.; Arai, Y. Kinetics of competitive adsorption/desorption of arsenate and phosphate at the ferrihydrite–water interface. Chem. Geol. 2014, 368, 31–38.
- Rivas-Pérez, I.M.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As (V) and P competitive sorption on soils, by-products and waste materials. Int. J. Environ. Res. Public Health 2015, 12, 15706–15715.
- Senn, A.-C.; Hug, S.J.; Kaegi, R.; Hering, J.G.; Voegelin, A. Arsenate co-precipitation with Fe(II) oxidation products and retention or release during precipitate aging. Water Res. 2018, 131, 334–345.
- Anawar, H.M.; Rengel, Z.; Damon, P.; Tibbett, M. Arsenic-phosphorus interactions in the soil-plant-microbe system: Dynamics of uptake, suppression and toxicity to plants. Environ. Pollut. 2018, 233, 1003–1012.
- Sanz, E.; Munoz-Olivas, R.; Camara, C.; Sengupta, M.K.; Ahamed, S. Arsenic speciation in rice, straw, soil, hair and nails samples from the arsenic-affected areas of Middle and Lower Ganga plain. J. Environ. Sci. Health Part A 2007, 42, 1695–1705.
- Tripathi, R.; Tripathi, P.; Dwivedi, S.; Dubey, S.; Chakrabarty, D.; Trivedi, P. Arsenomics: Omics of arsenic metabolism in plants. Front. Physiol. 2012, 3, 275.
- Lambkin, D.; Alloway, B. Arsenate-induced phosphate release from soils and its effect on plant phosphorus. Water Air Soil Pollut. 2003, 144, 41–56.
- Quader, I.S.B. Effect of Arsenic, Nitrogen and Phosphorus on Growth and Yield of Rice. Doctoral Dissertation, Department of Soil Science, Sher-e-Bangla Agricultural University, Dhaka, Bangladesh, 2017.
- Armstrong, W. Oxygen diffusion from the roots of some British bog plants. Nature 1964, 204, 801–802.
- Bolan, N.; Mahimairaja, S.; Kunhikrishnan, A.; Choppala, G. Phosphorus–arsenic interactions in variable-charge soils in relation to arsenic mobility and bioavailability. Sci. Total Environ. 2013, 463, 1154–1162.
- Fitz, W.J.; Wenzel, W.W. Arsenic transformations in the soil–rhizosphere–plant system: Fundamentals and potential application to phytoremediation. J. Biotechnol. 2002, 99, 259–278.
- Neupane, G.; Donahoe, R.J. Calcium–phosphate treatment of contaminated soil for arsenic immobilization. Appl. Geochem. 2013, 28, 145–154.
- Szegedi, K.; Vetterlein, D.; Jahn, R. Modelling rhizosphere transport in the presence of goethite, including competitive uptake of phosphate and arsenate. Plant Soil 2010, 330, 481–501.
- Peryea, F. Phosphate starter fertilizer temporarily enhances soil arsenic uptake by apple trees grown under field conditions. Hortscience 1998, 33, 826–829.
- Geng, C.-N.; Zhu, Y.-G.; Liu, W.-J.; Smith, S.E. Arsenate uptake and translocation in seedlings of two genotypes of rice is affected by external phosphate concentrations. Aquat. Bot. 2005, 83, 321–331.
- Lee, C.-H.; Wu, C.-H.; Syu, C.-H.; Jiang, P.-Y.; Huang, C.-C.; Lee, D.-Y. Effects of phosphorous application on arsenic toxicity to and uptake by rice seedlings in As-contaminated paddy soils. Geoderma 2016, 270, 60–67.
- Zou, Q.; Liu, F.; Yang, J.-H. Adsorption-desorption and competitive adsorption of arsenic and phosphorus in purple soil. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2009, 20, 1383–1389.
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994.
- Bissen, M.; Frimmel, F.H. Arsenic—A review. Part I: Occurrence, toxicity, speciation, mobility. Acta Hydrochim. Hydrobiol. 2003, 31, 9–18.
- Kubicki, J.D. Comparison of As (III) and As (V) complexation onto Al-and Fe-hydroxides. Adv. Arsen. Res. Integr. Exp. Obs. Stud. Implic. Migr. 2005, 915, 104–117.
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995.
- Manning, B.A.; Goldberg, S. Modeling competitive adsorption of arsenate with phosphate and molybdate on oxide minerals. Soil Sci. Soc. Am. J. 1996, 60, 121–131.
- Violante, A.; Pigna, M. Competitive sorption of arsenate and phosphate on different clay minerals and soils. Soil Sci. Soc. Am. J. 2002, 66, 1788–1796.
- Vetterlein, D.; Szegedi, K.; Ackermann, J.; Mattusch, J.; Neue, H.U.; Tanneberg, H.; Jahn, R. Competitive mobilization of phosphate and arsenate associated with goethite by root activity. J. Environ. Qual. 2007, 36, 1811–1820.
- Koo, N.; Kim, M.-S.; Hyun, S.; Kim, J.-G. Effects of the incorporation of phosphorus and iron into arsenic-spiked artificial soils on root growth of lettuce using response surface methodology. Commun. Soil Sci. Plant Anal. 2013, 44, 1259–1271.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
16 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




