Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Choukri Ben Mamoun | + 2737 word(s) | 2737 | 2021-09-08 05:28:14 | | | |
| 2 | Rita Xu | Meta information modification | 2737 | 2021-09-16 04:41:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ben Mamoun, C. Treatment of Human Babesiosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/14222 (accessed on 07 February 2026).
Ben Mamoun C. Treatment of Human Babesiosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/14222. Accessed February 07, 2026.
Ben Mamoun, Choukri. "Treatment of Human Babesiosis" Encyclopedia, https://encyclopedia.pub/entry/14222 (accessed February 07, 2026).
Ben Mamoun, C. (2021, September 15). Treatment of Human Babesiosis. In Encyclopedia. https://encyclopedia.pub/entry/14222
Ben Mamoun, Choukri. "Treatment of Human Babesiosis." Encyclopedia. Web. 15 September, 2021.
Copy Citation
Babesiosis is an emerging tick-borne disease caused by apicomplexan parasites of the genus Babesia. With its increasing incidence worldwide and the risk of human-to-human transmission through blood transfusion, babesiosis is becoming a rising public health concern.
babesiosis
Babesia microti
Babesia duncani
parasite
therapy
atovaquone
endochin-like quinolones (ELQs)
1. Introduction
Human babesiosis is a rapidly emerging tick-born infectious disease caused by intraerythrocytic parasites of the genus Babesia. Of several hundred Babesia species identified so far, only a few are known to infect humans. These include Babesia microti, Babesia duncani, Babesia divergens and divergens-like species, Babesia crassa-like, and Babesia venatorum [1]. In the United States, most cases of human babesiosis have been attributed to infection with B. microti, but sporadic cases due to infection with B. duncani and B. divergens-like MO1 have also been reported. In Europe, B. divergens used to be the main species responsible for infection in humans. However, recent studies suggest that B. microti and B. venatorum are now more prevalent than B. divergens [2]. In China, human babesiosis is mainly caused by B. microti and B. venatorum, and in the rest of the world, only a few sporadic cases have been reported and were mostly linked to B. microti infection [2].
Babesia spp. are apicomplexan parasites that infect the host red blood cells and are transmitted to mammals by tick vectors (Figure 1). The species of ticks involved in the transmission of Babesia pathogens vary depending on the geographical area and parasite species [1][2]. During the life cycle of Babesia, humans are typically accidental hosts, and most infections are linked to a tick route of transmission [1][2]. However, an increasing number of transfusion-transmitted babesiosis cases have been reported in the US over the past 2–3 decades, making Babesia infections a major public health concern [1][3][4][5][6]. In 2011, human babesiosis became a nationally notifiable disease in the US [5] and as one of the most common transfusion-transmitted pathogens in the US, B. microti was added to the list of significant threats to the blood supply [3][4]. In addition to human-to-human transmission through blood transfusion, several reports have also established the possibility of transplacental transmission from mother to child [1].
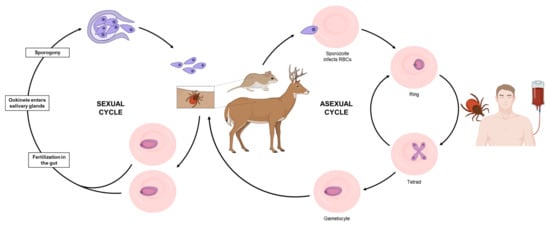
Figure 1. Cycle of transmission of the most common Babesia species, B. microti. During a blood meal, an infected tick introduces merozoites into the host (mouse or deer, for example). Free merozoites enter red blood cells and undergo asexual replication. While in the blood, some parasites differentiate into male and female gametocytes (not morphologically recognizable by light microscopy). These gametocytes are then taken up by a tick during a blood meal and differentiate into gametes. While in the gut, gametes fuse to form a zygote, that will subsequently undergo meiotic and several mitotic divisions to form sporozoites that are then transmitted to a mammalian host. Humans are typically accidental hosts and become infected through the bite of an infected tick. Human to human transmission is also possible via blood transfusion.
In most individuals, babesiosis remains asymptomatic or presents with mild flu-like symptoms [1][2]. However, in more susceptible populations, such as the elderly, asplenic, or immunocompromised individuals, the disease can become severe and even life-threatening, with symptoms such as severe anemia, acute respiratory distress, organ failure, and death [1][2].
2. Current Treatments against Human Babesiosis
The current arsenal for the treatment of human babesiosis relies principally on four drugs: atovaquone, azithromycin, clindamycin, and quinine. Atovaquone is used to treat several human diseases, including Pneumocystis jirovecii pneumonia [7], toxoplasmosis [8], and malaria (in combination with proguanil (Malarone) [9]. In apicomplexan parasites, atovaquone targets the cytochrome bc1 complex of the mitochondrial electron transport chain (Figure 2 and Figure 3) [10][11][12][13]. Azithromycin is a relatively broad-spectrum antibiotic indicated for the treatment of numerous bacterial infections, such as those caused by Staphylococcus spp. [14][15][16] and Legionella spp. [17]. The antibiotic is also used for the treatment of toxoplasmosis [12] and, in combination with other drugs, for the treatment of malaria [18]. Azithromycin is a well-characterized protein synthesis inhibitor, which in apicomplexan parasites targets the translation machinery in the apicoplast (Figure 2) [19][20][21]. It is worth noting that azithromycin was found to have a “delayed death” effect, in which parasite division produces viable daughter cells that are subsequently unable to divide in the following cycle [19][21][22]. Clindamycin is another antibiotic commonly used for the treatment of various bacterial infections [23] and repurposed for the treatment of parasitic infections. In combination with quinine, clindamycin is used for the treatment of both malaria and babesiosis [24][25][26]. Several reports have suggested that clindamycin acts in a similar way as azithromycin and targets protein synthesis in the apicoplast (Figure 2) [19][21][22]. Furthermore, selection of clindamycin-resistant T. gondii parasites showed cross-resistance to azithromycin, further suggesting a common target [27]. Quinine is a widely used antimalarial agent, typically administered in combination with an antibiotic such as clindamycin or doxycycline [28]. However, the drug is poorly tolerated and, as such, tends to be replaced by alternative drugs with fewer side-effects [28][29]. In malaria parasites, several modes of action for quinine have been proposed. The most commonly reported mechanism of action involves the disruption of hemozoin formation, resulting in accumulation of free ferriprotoporphyrin IX, a by-product of hemoglobin degradation, which is deleterious to parasite growth [30][31][32]. Confocal imaging using fluorescent derivatives of quinine and its structural analogues, quinidine and chloroquine, have shown accumulation of the probes in the digestive vacuole, consistent with the activity of this compound in this organelle [32][33]. Unlike Plasmodium parasites, Babesia species lack a digestive vacuole, do not degrade hemoglobin, and do not produce hemozoin. Therefore, the mode of action of quinine against Babesia parasites is likely to be different from that in Plasmodium. Interestingly, fluorescent probes were found to bind to phospholipids and to accumulate in membranous structures, including the parasite plasma membrane, the endoplasmic reticulum, and the mitochondrion, suggesting that quinine may inactivate specific biological functions in these organelles [32][33]. Another proposed hypothesis is that quinine acts as a DNA intercalator [34][35][36]. However, the lack of fluorescence in the nucleus reported by Woodland et al. seem to refute interactions with DNA as a potential mode of action [32][33]. More recently, a study in P. falciparum using thermal shift assays suggested that the purine nucleoside phosphorylase (PfPNP) might also be a target of quinine [37].
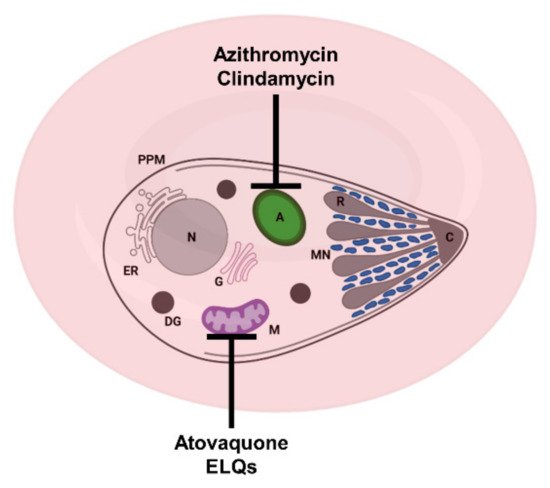
Figure 2. Schematic representation of a Babesia-infected red blood cell and sites of action of some approved and experimental drugs. Azithromycin and clindamycin target the apicoplast; atovaquone and ELQs target the mitochondrion. A: apicoplast, C: conoid + polar rings, DG: dense granule, ER: endoplasmic reticulum, G: Golgi apparatus, M: mitochondrion, MN: microneme, PPM: parasite plasma membrane and R: rhoptry.
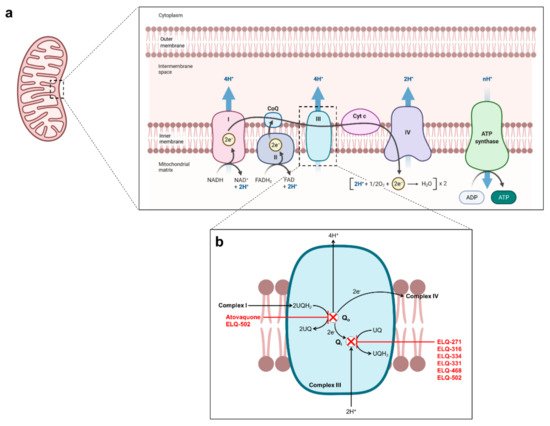
Figure 3. Proposed mechanism of action of atovaquone and endochin-like quinolones in Babesia mitochondrion. (a) Schematic representation the mitochondrial electron transfer chain. (b) Schematic representation of the parasite bc1 complex with proposed mode of action of atovaquone and ELQs.
The severity of babesiosis depends mainly on the host’s immune status, the presence of risk factors and the Babesia species responsible for the infection. In symptomatic patients, babesiosis usually manifests with flu-like symptoms such as fever, fatigue, chills, sweats, and headache [38]. For this moderate form of the disease, typically associated with a low parasitemia level (<4%) [26], no hospital admission is required and a 7–10-day treatment course of oral atovaquone + azithromycin (500 mg azithromycin on day 1, followed by 250 mg on subsequent days + 750 mg b.i.d. atovaquone) is recommended [26][38]. Babesiosis typically resolves within seven days from the start of the treatment, but asymptomatic, low level parasitemia may persist for up to one year [26]. Monitoring of persistent parasitemia in immunocompetent individuals following treatment is usually not necessary. However, given the risk of transmission of Babesia parasites through blood transfusion, these patients are excluded as blood donors [3]. Immunocompromised individuals are more at risk of developing a severe form of babesiosis, resulting in complications such as acute respiratory distress syndrome, disseminated intravascular coagulation, severe hemolytic anemia, organ failure, splenic rupture, relapse, and death [2][26]. A combination of oral clindamycin + quinine (600 mg + 650 mg, every 8 h) is the standard of care for the treatment of severe babesiosis [26][38]. However, this treatment regimen is frequently associated with serious side effects, such as hearing loss, vertigo, and tinnitus. In some cases, these side effects can be so severe that dose reduction or discontinuation of treatment is required [38]. Recently, it has been demonstrated that a combination of atovaquone + azithromycin is also suitable for the treatment of severe babesiosis, displaying comparable efficacy to clindamycin + quinine with fewer side effects [39]. Although atovaquone + azithromycin is now the preferred course of treatment for severe babesiosis, the standard 7–10-day treatment regimen of oral atovaquone + azithromycin is usually not enough to eliminate Babesia infection. Higher doses, longer treatment duration, and in some cases intravenous administration is required to clear the infection [26]. It is also worth noting that the use of immunosuppressive agents such as Rituximab to treat prior illnesses (B cell lymphoid malignancies, rheumatoid arthritis, etc.) may lead to babesiosis relapse and extended persistence of Babesia parasites [40][41][42].
One downside of a prolonged treatment regimen and dose escalation is the risk of developing drug resistance. Previous reports have established the emergence of mutations in the cytochrome b (Cytb) of Babesia parasites in humans and animal models following treatment with atovaquone [11][42][43]. In 2016, Lemieux et al. examined clinical isolates of relapsing babesiosis and identified a methionine to isoleucine mutation (M134I) in the Qo site (atovaquone-binding site) of the BmCytb [43]. This same mutation was observed in a murine model of B. microti infection [11], as well as in other apicomplexan parasites, such as P. falciparum and T. gondii [43]. Later, Simon et al. reported a Y272C mutation in the BmCytb Qo site in a patient presenting with relapsed B. microti infection following an atovaquone + azithromycin treatment course [42]. In both cases, these mutations have been shown to impact the atovaquone-binding domain [44] and appear to be associated with decreased sensitivity to the drug [42][43]. With regard to azithromycin resistance, sequencing of clinical isolates obtained from patients with relapsing babesiosis identified mutations in the ribosomal protein subunit L4 (RPL4) encoded by the apicoplast genome [42][43]. Lemieux et al. identified three substitutions in the RPL4: R86H, R86C and S73L [43]. Simon et al. observed the same R86C mutation in a patient presenting with relapsing babesiosis following atovaquone + azithromycin treatment [42]. Similar mutations associated with azithromycin resistance have been reported in P. falciparum [20] and S. pneumoniae [45] RPL4. Alternative management strategies for human babesiosis in the case of persistent relapse include the use of different drug combinations such as atovaquone + azithromycin + clindamycin, atovaquone + clindamycin, atovaquone + proguanil, or atovaquone + azithromycin + clindamycin + quinine [26][41][46][47]. The introduction of other drugs such as doxycycline, moxifloxacin, pentamidine, trimethoprim-sulfamethoxazole or artemisinin to treatment regimens with the standard therapies was also reported [40][48]. A recent study in a small cohort of patients suffering from Lyme disease and babesiosis co-infection suggested improvement, and in some cases remission, following one course of disulfiram monotherapy [49]. In patients with high parasitemia (>10%), exchange transfusion is recommended and often results in a rapid reduction of the parasite load [26][50].
Despite clinical evidence that atovaquone, azithromycin, clindamycin and quinine can be used to manage human babesiosis, preclinical evaluation of these drugs in different models of Babesia infection has not demonstrated unanimous results with regards to their efficacy. Clindamycin showed only limited activity at a dose of 300 mg/kg (p.o.) in B. microti-infected Mongolian jirds [51]. When evaluated in B. microti-infected hamster, a course of 150 mg/kg (i.m. or p.o.) of clindamycin resulted in a two-fold decrease in peak parasitemia. Similar results were obtained when clindamycin was administered in combination with quinine [52]. AbouLaila et al. reported a ~three-fold decrease in peak parasitemia following i.p. injection of 500 mg/kg of clindamycin in B. microti-infected Balb/c mice [53]. Another study using the same Balb/c model of B. microti infection showed that oral administration of clindamycin at 25, 50, and 100 mg/kg did not lead to reduction of parasite burden [54]. Similar results were obtained by Lawres et al. following oral administration of 10 or 50 mg/kg of clindamycin to immunocomprimized mice infected with B. microti [11]. The consensus seems to be more apparent in the case of quinine, where most studies report no effect on parasitemia following administration of quinine as a single drug [11][52][54]. Interestingly, a combination of clindamycin + quinine was reported to achieve up to 70% suppression of parasitemia [55] and result in a faster resolution of parasitemia compared to clindamycin alone [52], suggesting a potential synergy between the two drugs. Preclinical investigation of azithromycin efficiency against Babesia parasites also yielded inconsistent results. In B. microti-infected Balb/c mice, a four-day treatment course with azithromycin at 25, 50, and 100 mg/kg was found to be potent, resulting in 75–96% suppression of parasitemia [54]. In contrast, the evaluation of azithromycin in B. microti-infected SCID mice showed no effect on parasitemia at 10 and 50 mg/kg after a seven-day treatment course [11]. Similar results were obtained in B. microti-infected hamsters, where 150 mg/kg azithromycin treatment regimen, administered daily for almost two weeks, showed no apparent effect on parasitemia [56]. Out of the four clinically used drugs in the treatment of babesiosis, only atovaquone seems to consistently show high potency against Babesia parasites [11][56][57][58][59]. Studies carried out in B. microti-infected hamsters and SCID mice reported fast clearance of parasitemia following treatment with atovaquone [11][56]. However, recrudescence due to atovaquone-resistant parasites was observed [11][56]. In B. microti-infected hamsters, a combination therapy of atovaquone + azithromycin resulted in rapid clearance of parasitemia without recrudescence [56]. In a lethal model of B. microti infection in hamsters, atovaquone monotherapy was found to be superior to a combination of clindamycin + quinine, resulting in low to undetectable parasitemia and extended survival [58]. Potency of atovaquone was also demonstrated in B. divergens [59] and B. duncani [57] models, with IC50 values in the low nanomolar range. In gerbils, although prophylaxis experiments were not successful, a dose of atovaquone as low as 0.5 mg/kg was found to efficiently prevent B. divergens infection, so long as daily treatment was maintained several days post-infection [59]. In the case of B. duncani, a treatment course of 10 mg/kg atovaquone resulted in a clear reduction of parasitemia and 80% survival using a mouse model of lethal infection [57]. The results derived from the evaluation of atovaquone, azithromycin, clindamycin, and quinine in preclinical models of babesiosis are summarized in Table 1.
While combinations of atovaquone + azithromycin and clindamycin + quinine have been used for more than 20 years for the treatment of human babesiosis [60], the efficacy of these drugs and their primary modes of action in Babesia parasites have only recently started to be elucidated.
Table 1. Reported efficacy of atovaquone, azithromycin, clindamycin and quinine in animal models of babesiosis.
| Drug | Treatment Regimen | Model | Effect | Ref. |
|---|---|---|---|---|
| Atovaquone | 20 mg/kg (p.o.), 5 d | B. microti Balb/c mice |
~5.7 × reduction in peak parasitemia. | [61] |
| 25 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
77% suppression of parasitemia at DPI 9. | [54] | |
| 50 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
87% suppression of parasitemia at DPI 9. | [54] | |
| 100 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
93% suppression of parasitemia at DPI 9. | [54] | |
| 10 mg/kg (p.o.), 7 d | B. microti SCID mice |
Parasitemia clearance followed by recrudescence by D5-9 post-treatment. | [11] | |
| 10 mg/kg (p.o.), 10 d | B. microti SCID mice |
Parasitemia clearance followed by recrudescence by D14 post-treatment. | [57] | |
| 10 mg/kg (p.o.), 10 d | B. duncani C3H/HeJ mice |
Parasitemia clearance followed by recrudescence by D10 post-treatment. 80% survival. | [57] | |
| Azithromycin | 25 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
75% suppression of parasitemia at DPI 9. | [54] |
| 50 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
96% suppression of parasitemia at DPI 9. | [54] | |
| 100 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
95% suppression of parasitemia at DPI 9. | [54] | |
| 10 mg/kg (p.o.), 7 d | B. microti SCID mice |
No effect. | [11] | |
| 50 mg/kg (p.o.), 7 d | B. microti SCID mice |
No effect. | [11] | |
| Clindamycin | 300 mg/kg (p.o.), 5d | B. microti Mongolian jirds |
9.4% suppression of parasitemia at DPI 9. | [51] |
| 150 mg/kg (i.m.), 8d | B. microti Golden hamsters |
~2× reduction in peak parasitemia. | [52] | |
| 150 mg/kg (p.o.), 8d | B. microti Golden hamsters |
~2× reduction in peak parasitemia. | [52] | |
| 500 mg/kg (i.p.), 5d | B. microti Balb/c mice |
~3.2× reduction in peak parasitemia. | [53] | |
| 25 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
No effect. | [54] | |
| 50 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
No effect. | [54] | |
| 100 mg/kg (p.o.), 4 d | B. microti Balb/c mice | No effect. | [54] | |
| 10 mg/kg (p.o.), 7 d | B. microti SCID mice |
No effect. | [11] | |
| 50 mg/kg (p.o.), 7 d | B. microti SCID mice |
No effect. | [11] | |
| Quinine | 125 mg/kg (s.c.), 8d | B. microti Golden hamsters |
No effect. | [52] |
| 250 mg/kg (p.o.), 8d | B. microti Golden hamsters |
No effect. | [52] | |
| 25 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
No effect. | [54] | |
| 50 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
No effect. | [54] | |
| 100 mg/kg (p.o.), 4 d | B. microti Balb/c mice |
No effect. | [54] | |
| 10 mg/kg (p.o.), 7 d | B. microti SCID mice |
No effect. | [11] | |
| 50 mg/kg (p.o.), 7 d | B. microti SCID mice |
No effect. | [11] | |
| 100 mg/kg (p.o.), 7 d | B. microti SCID mice |
No effect. | [11] |
References
- Krause, P.J. Human babesiosis. Int. J. Parasit. 2019, 49, 165–174.
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, 34.
- Levin, A.E.; Krause, P.J. Transfusion-transmitted babesiosis: Is it time to screen the blood supply? Curr. Opin. Hematol. 2016, 23, 573–580.
- Lobo, C.A.; Singh, M.; Rodriguez, M. Human babesiosis: Recent advances and future challenges. Curr. Opin. Hematol. 2020, 27, 399–405.
- Moritz, E.D.; Winton, C.S.; Tonnetti, L.; Townsend, R.L.; Berardi, V.P.; Hewins, M.E.; Weeks, K.E.; Dodd, R.Y.; Stramer, S.L. Screening for Babesia microti in the U. S. Blood Supply. N. Engl. J. Med. 2016, 375, 2236–2245.
- Tonnetti, L.; Townsend, R.L.; Dodd, R.Y.; Stramer, S.L. Characteristics of transfusion-transmitted Babesia microti, American Red Cross 2010–2017. Transfusion 2019, 59, 2908–2912.
- Mantadakis, E. Pneumocystis jirovecii Pneumonia in Children with Hematological Malignancies: Diagnosis and Approaches to Management. J. Fungi 2020, 6, 331.
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17.
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial pharmacology and therapeutics of atovaquone. J. Antimicrob. Chemother. 2013, 68, 977–985.
- Jacobsen, L.; Husen, P.; Solov’yov, I.A. Inhibition Mechanism of Antimalarial Drugs Targeting the Cytochrome bc1 Complex. J. Chem. Inf. Modeling 2021, 61, 1334–1345.
- Lawres, L.A.; Garg, A.; Kumar, V.; Bruzual, I.; Forquer, I.P.; Renard, I.; Virji, A.Z.; Boulard, P.; Rodriguez, E.X.; Allen, A.J.; et al. Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J. Exp. Med. 2016, 213, 1307–1318.
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Shahdin, S.; Daryani, A. Activities of anti-Toxoplasma drugs and compounds against tissue cysts in the last three decades (1987 to 2017), a systematic review. Parasitol. Res. 2018, 117, 3045–3057.
- Vaidya, A.B.; Mather, M.W. Atovaquone resistance in malaria parasites. Drug Resist. Updates 2000, 3, 283–287.
- Daniel, R. Azithromycin, erythromycin and cloxacillin in the treatment of infections of skin and associated soft tissues. European Azithromycin Study Group. J. Int. Med. Res. 1991, 19, 433–445.
- Dinwiddie, R. Anti-inflammatory therapy in cystic fibrosis. J. Cyst. Fibros. 2005, 4 (Suppl. S2), 45–48.
- Ladhani, S.; Garbash, M. Staphylococcal skin infections in children: Rational drug therapy recommendations. Paediatr. Drugs 2005, 7, 77–102.
- Carratala, J.; Garcia-Vidal, C. An update on Legionella. Curr. Opin. Infect. Dis. 2010, 23, 152–157.
- Van Eijk, A.M.; Terlouw, D.J. Azithromycin for treating uncomplicated malaria. Cochrane Database Syst. Rev. 2011, 2011, Cd006688.
- Chakraborty, A. Understanding the biology of the Plasmodium falciparum apicoplast; an excellent target for antimalarial drug development. Life Sci. 2016, 158, 104–110.
- Sidhu, A.B.S.; Sun, Q.G.; Nkrumah, L.J.; Dunne, M.W.; Sacchettini, J.C.; Fidock, D.A. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J. Biol. Chem. 2007, 282, 2494–2504.
- Beckers, C.J.M.; Roos, D.S.; Donald, R.G.K.; Luft, B.J.; Schwab, J.C.; Cao, Y.; Joiner, K.A. Inhibition of cytoplasmic and organellar protein synthesis in Toxoplasma gondii. Implications for the target of macrolide antibiotics. J. Clin. Investig. 1995, 95, 367–376.
- Dahl, E.L.; Rosenthal, P.J. Multiple antibiotics exert delayed effects against the Plasmodium falciparum anicoplast. Antimicrob. Agents Chemother. 2007, 51, 3485–3490.
- Murphy, P.B.; Bistas, K.G.; Le, J.K. Clindamycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Griffith, K.S.; Lewis, L.S.; Mali, S.; Parise, M.E. Treatment of malaria in the United States: A systematic review. Jama 2007, 297, 2264–2277.
- Lell, B.; Kremsner, P.G. Clindamycin as an antimalarial drug: Review of clinical trials. Antimicrob. Agents Chemother. 2002, 46, 2315–2320.
- Smith, R.P.; Hunfeld, K.P.; Krause, P.J. Management strategies for human babesiosis. Expert Rev. Anti-Infect. Ther. 2020, 18, 625–636.
- Pfefferkorn, E.R.; Borotz, S.E. Comparison of mutants of Toxoplasma gondii selected for resistance to azithromycin, spiramycin, or clindamycin. Antimicrob. Agents Chemother. 1994, 38, 31–37.
- Talapko, J.; Škrlec, I.; Alebić, T.; Jukić, M.; Včev, A. Malaria: The Past and the Present. Microorganisms 2019, 7, 179.
- Tse, E.G.; Korsik, M.; Todd, M.H. The past, present and future of anti-malarial medicines. Malar. J. 2019, 18, 93.
- Sullivan, D.J., Jr.; Gluzman, I.Y.; Russell, D.G.; Goldberg, D.E. On the molecular mechanism of chloroquine’s antimalarial action. Proc. Natl. Acad. Sci. USA 1996, 93, 11865–11870.
- Tang, Y.Q.; Ye, Q.; Huang, H.; Zheng, W.Y. An Overview of Available Antimalarials: Discovery, Mode of Action and Drug Resistance. Curr. Mol. Med. 2020, 20, 583–592.
- Woodland, J.G.; Hunter, R.; Smith, P.J.; Egan, T.J. Shining new light on ancient drugs: Preparation and subcellular localisation of novel fluorescent analogues of Cinchona alkaloids in intraerythrocytic Plasmodium falciparum. Org. Biomol. Chem. 2017, 15, 589–597.
- Woodland, J.G.; Hunter, R.; Smith, P.J.; Egan, T.J. Chemical Proteomics and Super-resolution Imaging Reveal That Chloroquine Interacts with Plasmodium falciparum Multidrug Resistance-Associated Protein and Lipids. ACS Chem. Biol. 2018, 13, 2939–2948.
- Punihaole, D.; Workman, R.J.; Upadhyay, S.; Van Bruggen, C.; Schmitz, A.J.; Reineke, T.M.; Frontiera, R.R. New Insights into Quinine-DNA Binding Using Raman Spectroscopy and Molecular Dynamics Simulations. J. Phys. Chem. B 2018, 122, 9840–9851.
- Golan, D.E.; Armstrong, E.J.; Armstrong, A.W.; Tashjian, A.H. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 1–1956.
- Percário, S.; Moreira, D.R.; Gomes, B.A.; Ferreira, M.E.; Gonçalves, A.C.; Laurindo, P.S.; Vilhena, T.C.; Dolabela, M.F.; Green, M.D. Oxidative stress in malaria. Int. J. Mol. Sci. 2012, 13, 16346–16372.
- Dziekan, J.M.; Yu, H.; Chen, D.; Dai, L.; Wirjanata, G.; Larsson, A.; Prabhu, N.; Sobota, R.M.; Bozdech, Z.; Nordlund, P. Identifying purine nucleoside phosphorylase as the target of quinine using cellular thermal shift assay. Sci. Transl. Med. 2019, 11.
- Krause, P.J.; Auwaerter, P.G.; Bannuru, R.R.; Branda, J.A.; Falck-Ytter, Y.T.; Lantos, P.M.; Lavergne, V.; Meissner, H.C.; Osani, M.C.; Rips, J.G.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA): 2020 Guideline on Diagnosis and Management of Babesiosis. Clin. Infect. Dis. 2021, 72, 185–189.
- Kletsova, E.A.; Spitzer, E.D.; Fries, B.C.; Marcos, L.A. Babesiosis in Long Island: Review of 62 cases focusing on treatment with azithromycin and atovaquone. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 7.
- Krause, P.J.; Gewurz, B.E.; Hill, D.; Marty, F.M.; Vannier, E.; Foppa, I.M.; Furman, R.R.; Neuhaus, E.; Skowron, G.; Gupta, S.; et al. Persistent and relapsing babesiosis in Immunocompromised patients. Clin. Infect. Dis. 2008, 46, 370–376.
- Raffalli, J.; Wormser, G.P. Persistence of babesiosis for >2 years in a patient on rituximab for rheumatoid arthritis. Diagn. Microbiol. Infect. Dis. 2016, 85, 231–232.
- Simon, M.S.; Westblade, L.F.; Dziedziech, A.; Visone, J.E.; Furman, R.R.; Jenkins, S.G.; Schuetz, A.N.; Kirkman, L.A. Clinical and Molecular Evidence of Atovaquone and Azithromycin Resistance in Relapsed Babesia microti Infection Associated with Rituximab and Chronic Lymphocytic Leukemia. Clin. Infect. Dis. 2017, 65, 1222–1225.
- Lemieux, J.E.; Tran, A.D.; Freimark, L.; Schaffner, S.F.; Goethert, H.; Andersen, K.G.; Bazner, S.; Li, A.; McGrath, G.; Sloan, L.; et al. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat. Microbiol. 2016, 1, 7.
- Birth, D.; Kao, W.C.; Hunte, C. Structural analysis of atovaquone-inhibited cytochrome bc(1) complex reveals the molecular basis of antimalarial drug action. Nat. Commun. 2014, 5, 11.
- Doktor, S.Z.; Shortridge, V.D.; Beyer, J.M.; Flamm, R.K. Epidemiology of macrolide and/or lincosamide resistant Streptococcus pneumoniae clinical isolates with ribosomal mutations. Diagn. Microbiol. Infect. Dis. 2004, 49, 47–52.
- Li, Y.J.; Stanley, S.; Villalba, J.A.; Nelson, S.; Gelfand, J. Case Report: Overwhelming Babesia Parasitemia Successfully Treated Promptly with RBC Apheresis and Triple Therapy with Clindamycin, Azithromycin, and Atovaquone. Open Forum Infect. Dis. 2020, 7, 3.
- Vyas, J.M.; Telford, S.R.; Robbins, G.K. Treatment of refractory Babesia microti infection with atovaquone-proguanil in an HIV-infected patient: Case report. Clin. Infect. Dis. 2007, 45, 1588–1590.
- Man, S.Q.; Qiao, K.; Cui, J.; Feng, M.; Fu, Y.F.; Cheng, X.J. A case of human infection with a novel Babesia species in China. Infect. Dis. Poverty 2016, 5, 6.
- Gao, J.C.; Gong, Z.D.; Montesano, D.; Glazer, E.; Liegner, K. “Repurposing” Disulfiram in the Treatment of Lyme Disease and Babesiosis: Retrospective Review of First 3 Years’ Experience in One Medical Practice. Antibiotics 2020, 9, 868.
- Radcliffe, C.; Krause, P.J.; Grant, M. Repeat exchange transfusion for treatment of severe babesiosis. Transfus. Apher. Sci. 2019, 58, 638–640.
- Ruebush, T.K.; Contacos, P.G.; Steck, E.A. Chemotherapy of Babesia microti infections in Mongolian Jirds. Antimicrob. Agents Chemother. 1980, 18, 289–291.
- Rowin, K.S.; Tanowitz, H.B.; Wittner, M. Therapy of Experimental Babesiosis. Ann. Intern. Med. 1982, 97, 556–558.
- AbouLaila, M.; Munkhjargal, T.; Sivakumar, T.; Ueno, A.; Nakano, Y.; Yokoyama, M.; Yoshinari, T.; Nagano, D.; Katayama, K.; El-Bahy, N.; et al. Apicoplast-Targeting Antibacterials Inhibit the Growth of Babesia Parasites. Antimicrob. Agents Chemother. 2012, 56, 3196–3206.
- Yao, J.M.; Zhang, H.B.; Liu, C.S.; Tao, Y.; Yin, M. Inhibitory effects of 19 antiprotozoal drugs and antibiotics on Babesia microti infection in BALB/c mice. J. Infect. Dev. Ctries. 2015, 9, 1004–1010.
- Marley, S.E.; Eberhard, M.L.; Steurer, F.J.; Ellis, W.L.; McGreevy, P.B.; Ruebush, T.K. Evaluation of selected antiprotozoal drugs in the Babesia microti-hamster model. Antimicrob. Agents Chemother. 1997, 41, 91–94.
- Wittner, M.; Lederman, J.; Tanowitz, H.B.; Rosenbaum, G.S.; Weiss, L.M. Atovaquone in the treatment of Babesia microti infections in hamsters. Am. J. Trop. Med. Hyg. 1996, 55, 219–222.
- Chiu, J.E.; Renard, I.; Pal, A.C.; Singh, P.; Vydyam, P.; Thekkiniath, J.; Kumar, M.; Gihaz, S.; Pou, S.; Winter, R.W.; et al. Effective Therapy Targeting Cytochrome bc(1) Prevents Babesia Erythrocytic Development and Protects from Lethal Infection. Antimicrob. Agents Chemother. 2021, 65, AAC-00662.
- Hughes, W.T.; Oz, H.S. Successful Prevention and Treatment of Babesiosis with Atovaquone. J. Infect. Dis. 1995, 172, 1042–1046.
- Pudney, M.; Gray, J.S. Therapeutic efficacy of atovaquone against the bovine intraerythrocytic parasite, Babesia divergens. J. Parasitol. 1997, 83, 307–310.
- Krause, P.J. Babesiosis. Med. Clin. North. Am. 2002, 86, 361–373.
- Beshbishy, A.M.; Batiha, G.E.S.; Alkazmi, L.; Nadwa, E.; Rashwan, E.; Abdeen, A.; Yokoyama, N.; Igarashi, I. Therapeutic Effects of Atranorin towards the Proliferation of Babesia and Theileria Parasites. Pathogens 2020, 9, 127.
More
Information
Subjects:
Infectious Diseases
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
16 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




