Study of reproductive biology and pollination ecology helps in understanding the life history patterns of species. Such a study brings to light the bottlenecks, if any, on account of which the individuals of the species are not able to reproduce in nature and ultimately helps in planning appropriate conservation strategies for the species under threat. The present study was aimed at examining the morphological and reproductive variance in Berberis lycium, a threatened ecological specialist growing within shrubberies and open hillsides of the North-Western Himalayas in India. B. lycium displays three different variants. Flowering period ranges from February to September. Pollen viability as reported on fluorescein diacetate and acetocarmine treatments was highest for variant I, while maximum pollen output was obtained for variant III. Pollen pistil interaction is brought by the movement of anther towards stigma. Fluorescence microscopy of hand pollinated club shaped stigma shows that the germinating pollen form a ring over the receptive adaxial surface. Pollination syndrome is entomophily. Variant II attracts a significantly large number of pollinators from diverse insect families. Breeding experiments reflect that plants are self-compatible and cross fertile. Reproductive output (% fruit set) was highest for variant II followed by III and I, respectively.
1. Introduction
The entire Himalayan region is a repository of high value medicinal plants and is therefore, considered as a global biodiversity hotspot. Out of 18,440 plant species found in the Himalayan region, 8298 species are medicinally important
[1][2].
Berberis lycium is one among them. Commonly known as Indian barberry, it is an evergreen shrub belonging to family Berberidaceae. It is a sub-erect branched shrub growing to a height of 3–4 m. It is native to Nepal and is abundantly distributed in the Himalayas extending through West of Pakistan, North and Central India, Afghanistan and the entirety of Nepal
[3]. Within India, it is frequently distributed in Jammu and Kashmir, Uttar Pradesh, Madhya Pradesh, Himachal Pradesh, Uttrakhand, Sikkim and Nilgiri hills of Tamil Nadu within altitudinal gradients of 850–3500 m.a.s.l.
[3][4][5]. This plant is used in polyherbal formulation of organized medicine such as Ayurveda, Siddha and Unani
[6]. Pharmacological investigation of the entire plant body parts has revealed the presence of different secondary metabolites (alkaloids, tannins, saponins, flavonoids etc.) which signifies its potential role in the formulation of antibacterial, antifungal, antidiabetic and cardio-protective drugs
[7][8]. Active constituents include berberine
[9][10], an isoquinoline alkaloid which is widely known for its activity against severe diarrhea
[11], cholera
[12], latent malaria and amoebiasis
[12]. Owing to its wide spectrum of medicinal significance this plant is being exploited at an alarming rate. Besides, it is being eradicated from the Himalayan region to reclaim the hill slopes for agriculture or to extract a valuable drug berberidine from its roots and stem
[13][14]. Decline in the population of this species urges the need for its timely conservation so as to prevent this plant from getting critically endangered or extinct
[15].
To design an effective conservation strategy for any species of plants, information about its reproductive behavior and pollination ecology along with other threatening factors becomes imperative
[16][17]. Scientific data generated in such studies help in designing conservation strategies on one hand and in exploring the mechanism by which it has become endangered on the other
[18]. In this context, study of the reproductive biology of the plants, particularly endangered and overexploited assumes a paramount significance. Reproductive biology allows us to understand the different phenophases of a species and processes beginning from the production of gametes to the germination of the seeds and constrains that limit the multiplication of the species in nature
[19][20]. Studies on these aspects of reproductive biology of threatened taxa have provided valuable information regarding the limitation in the process of sexual reproduction and have helped in planning effective conservation and management programs
[20][21][22][23][24][25]. Mating and the breeding systems of plant species have found to be detrimental in designing the genetic structure of the populations. Pollination biology is an important parameter in the life history of flowering plants that determines the success of sexual reproduction
[26][27][28]. Effective conservation and management programmes based on the result of such studies have been executed for a number of threatened species
[29][30][31][32][33]. Such studies bring to light the bottlenecks, if any, on account of which the individuals of the species are not able to reproduce in nature
[34][35]. It also determines the genetic makeup of the populations, the magnitude of variations and therefore, ultimately helps in planning appropriate conservation strategies for the species under different categories of threat
[36][37].
Berberis lycium Royle is one of the heavily exploited and threatened medicinal plants of the Himalayan region. Every part of the plant is used for medicinal purposes
[38]. The species has been declared endangered in the North-West Himalayan region by FRLHT as per IUCN criteria of 2000 (envis.frlht.org/frlhtenvis.nic.in). Non-availability of basic biological data regarding phenology, reproductive biology, and pollination ecology of
Berberis lycium are a major concern and are detrimental to its recovery plans. To fill these gaps, the present study was conducted to explore and gather the information on
B. lycium with respect to above mentioned parameters, to understand this plant species at its various phenophases and to elucidate potential deficiencies in its reproduction and pollination mechanisms.
2. Phenology, Floral Organization and Traits
Flowers of Berberis lycium are bracteate, hypogynous, actinomorphic and hermaphrodite. Flowers grow in dense corymbose racemes. Flowers are bright, attractive and pale yellow in color, and grow in axillary clusters. Two porophylls are present over the calyx. Calyx in variants I and II is represented by 6 light yellow colored sepals in the two whorls of which the outer three are smaller than the inner three. In variant III calyx is represented by 2–3 whorls of sepals with number of sepals varying from 6 to 9. Corolla in variants I and II is bright yellow polypetalous having six petals. Corolla in variant III consists of 6–7 yellow colored petals. In flowers with 7 petals, as many as 7 stamens are present. Two nectaries are present at the base of each petal. Six antipetalous, adnate and bithecous stamens constitute the androecium in all the three variants. An anther has two lobes, each with two unequal small pollen sacs (SPSs) and large pollen sacs (LPSs). Gynoecium is represented by a single pistil which is differentiated into stigma, style and ovary. Stigma is round with a depression in the middle. It is wet and has a copious number of exudates. Style is hollow and short. Ovary is unilocular having three to five anatropous ovules. Number of ovules in variant III is usually 5 varying from 4 to 6. The fruits are berries, ovoid in shape and acquire purple or bright red color upon maturity. The size of berries may range from 8.2 ± 1.4 mm, 5.1 ± 1.1 mm to 6 ± 0.9 mm, 5.5 ± 1mm in length and diameter, respectively. Weight of the fruits range between 303 ± 24 and 209 ± 19 mg. Seeds are yellow to pinkish in color and on average the fruit contain 2–5 seeds (Figure 1).
Figure 1. Floral structure of
B. lycium (
A) Hermaphrodite flower (
B) Inner three larger sepals (
C) Outer three smaller sepals (
D) Two porophylls present over the calyx (
E) Two nectaries present at the base of each petal (
F) Anti-petalous, adnate and bithecous stamen (
G–
I)
Anatropous ovules (three, four and five).
2.1. Variant I
This is the predominant type observed. The plant bodies are differentiated into branched root and shoot system. Leaves arise alternately on the shoots in clusters. Their number varies from 3 to 9 per cluster. They are lanceolate with entire or spinous margins. Each node on the shoot bears one trifid spine. Inflorescences are racemose and arise from the axils of the leafy shoots in the last week of February. Peak flowering occurs in the middle of March. A bud takes about 20–22 days to transform into a flower after it appears on the inflorescence. Flowering completes by first week of April.
2.2. Variant II
These plants enter the reproductive phase in April and are marked by the presence of relatively long inflorescences. Leaves are papery and spathulate in shape with apical spine. Buds begin to open in the month of May in an acropetal succession in an inflorescence. Peak flowering was seen in the third week of May and completes by the second week of June. An inflorescence bears about 30 flowers on an average reaching up to 50 per inflorescence.
2.3. Variant III
Plants enter the reproductive phase in the month of September and continue producing flower till January. The leaves are either entire or with spines only towards the apical end of the lamina. Leaves arise in an alternate manner. Number of leaves at each node varies from 3 to 10. Leaves are also present on the inflorescences. Inflorescences of different sizes were present on a single plant ranging from 3 to 10 cm. Leaves are also present on the inflorescence. Number of sepals and petals varies ranges from 6 to 9 and 6 to 7, respectively. Number of ovules is usually 5. Detailed floral and morphological differences in different variants of B. lycium are provided in Table 1. One-way ANOVA showed that there was a significant difference in the majority of the morphological characters in different variants and the level of significance ranged from < 0.001 to <0.05. However, a number of other traits, such as width of the leaf, width of the bract, length and width of the porophylls, size of petal, length of stamens and width of flower, were not statistically different.
Table 1. Variability among three variants of Berberis lycium with respect to different floral traits.
| S. No. |
Parameter |
Variant I |
Variant II |
Variant III |
Mean Square |
F Value |
p-Value |
| 1 |
Time of flowering |
February |
April |
September |
39 |
3.96 |
<0.001 *** |
| 2 |
Length of leaf (cm) |
3.56 ± 0.25 |
4.36 ± 0.41 |
4.55 ± 0.31 |
0.8203 |
7.364 |
0.0243 * |
| 3 |
Width of leaf (cm) |
1.1 ± 0.2 |
0.8 ± 0.1 |
1 ± 0.1 |
0.07 |
3.5 |
0.0983 |
| 3 |
Length of inflorescence (cm) |
4.4 ± 0.7 |
11.5 ± 1.2 |
5.45 ± 1.2 |
44.02 |
37.45 |
<0.001 *** |
| 4 |
Number of flowers per inflorescence |
12 ± 4.04 |
30 ± 7.76 |
13 ± 4.16 |
356.8 |
11.39 |
<0.001 *** |
| 5 |
Diameter of flower (mm) |
7.2 ± 0.15 |
7 ± 0.2 |
8.7 ± 0.25 |
2.6133 |
61.9 |
<0.001 *** |
| 6 |
Percentage pollen viability |
93 ± 3.2 |
87 ± 3.7 |
86 ± 4 |
38.11 |
28.58 |
<0.001 *** |
| 7 |
Pollen output per flower |
8040 ± 372.6 |
6189 ± 454.5 |
8148 ± 552.9 |
2583222 |
11.86 |
0.00824 ** |
| 8 |
Length and width of the bract (mm) |
3.2 ± 0.25 × 2.3 ± 0.19 |
2.9 ± 0.21 × 2 ± 0.14 |
3.5 ± 0.27 × 1.9 ± 0.11 |
0.3733 |
8.842 |
0.0163 * |
| 9 |
Length and width of the porophylls (mm) |
2.5 ± 0.05 × 1.55 ± 0.11 |
1.9 ± 0.09 × 1.4 ± 0.13 |
2.2 ± 0.17 × 1.4 ± 0.09 |
0.05444 |
4.9 |
0.0548 |
| 10 |
Size of sepal’s outer whorl (mm) |
3.6 ± 0.51 × 3.2 ± 0.42 |
3.6 ± 0.81 × 3.3 ± 0.56 |
3.3 ± 0.31 × 2.9 ± 0.36 |
0.05778 |
4 |
0.0787 |
| 11 |
Size of sepal’s inner whorl (mm) |
5.8 ± 0.42 × 4.6 ± 0.51 |
6.2 ± 0.67 × 5.5 ± 0.62 |
5.6 ± 0.32 × 4.9 ± 0.55 |
0.00778 |
0.875 |
0.464 |
| 12 |
Size of petal (mm) |
5.75 ± 0.45 × 4.25 ± 0.41 |
6.25 ± 0.32 × 5.12 ± 0.56 |
6.15 ± 0.37 × 4.75 ± 0.31 |
0.09 |
5.4 |
0.0456 * |
| 13 |
Length of pistil (mm) |
4.55 ± 0.49 |
5.17 ± 0.47 |
4.95 ± 0.37 |
0.3333 |
13.04 |
0.00654 ** |
| 14 |
Length of stamen (mm) |
3.55 ± 0.49 |
3.67 ± 0.41 |
3.59 ± 0.39 |
0.2923 |
2.642 |
0.15 |
| 15 |
Width of flower (mm) |
7.3 ± 0.61 |
7.9 ± 0.47 |
7.7 ± 0.31 |
0.5983 |
5.543 |
0.0433 * |
3. Anthesis and Pattern of Flowering in Inflorescence
Flower anthesis starts when the petals of the flower start to unroll and separate from each other. This activity was seen during separate time periods for different variants. Flowers begin to open between 06:00 and 08:00 am and continue to open throughout the day with maximum number of flowers opening between 09:00 am and 04:00 pm. Increase in the mean day temperature results in early flower anthesis. In variant III, flowers buds start to open at 06:17 am followed by variant II at 07:00 am and variant I at 08:00 am, respectively. Flowers open randomly, exhibit dichogamy and are protogynous. On an average, a flower takes about 3 h to open completely (Table 2). Flowers continue to open throughout the day. None of the flowers open during the night. Anther dehiscence starts when the flowers are about 0.4–0.5 cm across. In some flowers, anthers dehisce once the flower has fully opened. Anther lobes dehisce sequentially one by one on alternate stamens. After about three days of pollination, the flowers start withering and on the fourth day, the fertilized red color pistil is exposed. Analysis of variance (ANOVA) showed that different variants differed significantly (p < 0.05) with respect to flowering, time and maximum anthesis while the other characters were statistically non-significant.
Table 2. Pattern of flower anthesis and anther dehiscence in Berberis lycium.
| Variant |
Anthesis |
Time |
Duration |
Maximum |
Completion |
Anther Dehiscence |
| I |
February |
08:00 am ± 17 min |
2.5 h ± 22 min |
76% ± 7.8 at 09:00–16:08 h |
Throughout day |
During and after dehiscence |
| II |
April |
07:00 am ± 24 min |
3 h ± 17 min |
82% ± 11.7 at 09:30–15:15 h |
Throughout day |
During and after dehiscence |
| III |
September |
06:17 am ± 13 min |
3.15 h ± 15 min |
79% ± 9.5 at 09:15–15:39 h |
Throughout day |
During and after dehiscence |
4. Stigma Receptivity
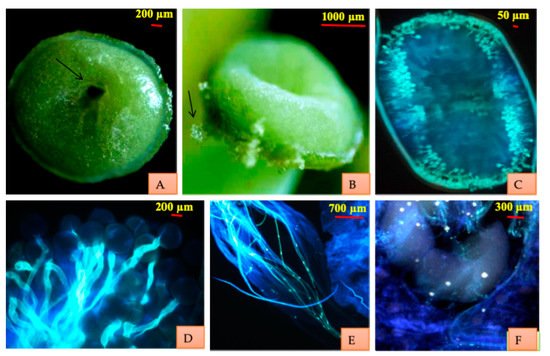
Fluorescence microscopy of the manually pollinated stigma (at different time intervals under Nikon Eclipse 80 Fluorescence microscope) reflects that stigma becomes receptive in the bud condition about three days before anthesis. It remains receptive for 26–28 h prior to anthesis. Stigma is green, wet and receptive on the day of anthesis. It is round with the depression in the middle. While still green in color, stigma loses receptivity after 28h of opening. Stigma acquires brown color 6–7 days after anthesis and is persistent. Fluorescence microscopy of the pistil showed the germinating pollen on the stigma, pollen tubes moving through the stylar tissue and entering the ovule through the micropyle (Figure 2). The pollen tubes reached the ovary within 30–40 min.
Figure 2. Stigmatic surface and receptivity. (
A) Stigma showing the depression in the middle which merges to hollow style. (
B) Pollen trapped on wet stigmatic surface. (
C) Pattern of pollen germination on the stigma. (
D) Pollen germination. (
E) Pollen tubes traversing through style. (
F) Pollen tubes entering the ovules.
5. Pollination Ecology
Hanging slide experiments rule out the possibility of wind pollination (anemophily). Berberis lycium is insect pollinated (entomophily). Insect visitors belong to 4 orders (Hymenoptera, Diptera, Lepidoptera and Coleoptera), 14 families and 17 genera. Hymenoptera was observed as the most dominant order with nine pollinator species (Figure 3). B. lycium has all the contrivances (brighter color, fragrance, nectar and pollen) for entomophily. Pollen deposition on the stigmatic surface requires the intervention of insects. Apis cerana and A. mellifera are predominant pollinators.
Figure 3. Insect visitors of Berberis lycium. (A) Eupeodes luniger; (B) Calliphora vomitoria; (C) Apis mellifera; (D) Calliphora sp.; (E) Dodona durga; (F) Heliophorus sena;(G) Sarcopha sp.; (H) Apis cerana; (I) Altica sp.; (J–L) Plecia spp. * Images captured with Nikon D7000 DSLR camera (Nikon, Tokyo, Japan).
When the proboscis of the nectar foraging insects touches the base of the stamen with the dehiscing anther, it pushes the stamen towards the stigma. This way the anther touches the stigmatic surface, thereby depositing a huge amount of pollen on the stigma. The anthers remain stuck at the stigma for 22–30 s and the stamen slowly retrieves back to its original position within 15 min. It was observed that the stamen with the dehiscing anther lobes gets pushed towards the stigma instantaneously thereby affecting the pollen deposition on the stigma. Pollinator abundance and richness were highest for variant I (Table 3).
Table 3. Insect visitors for different morphotypes of Berberis lycium and their foraging activity.
| S. No. |
Insect Visitor |
Order |
Family |
Variant I |
Variant II |
Variant III |
Foraging Activity |
| 1 |
Allograpta sp. |
Hymenoptera |
Apidae |
+ |
- |
+ |
Nectar |
| 2 |
Altica sp. |
Coleoptera |
Chrysomelidae |
+ |
- |
- |
Nectar and pollen |
| 3 |
Andrena flavipes |
Hymenoptera |
Adrenidae |
- |
+ |
- |
Nectar |
| 4 |
Aphidoidea (aphids) |
Hemiptera |
Aphididae |
+ |
- |
+ |
Pollen |
| 5 |
Apis cerana |
Hymenoptera |
Apidae |
+ |
+ |
+ |
Nectar |
| 6 |
Apis mellifera |
Hymenoptera |
Apidae |
+ |
+ |
+ |
Nectar |
| 7 |
Bombus trifasciatus |
Hymenoptera |
Apidae |
+ |
- |
+ |
Nectar and pollen |
| 8 |
Calliphora vomitoria |
Diptera |
Calliphoridae |
+ |
- |
- |
Nectar and pollen |
| 9 |
Camponotus pennsylvanicus |
Hymenoptera |
Formicidae |
+ |
- |
+ |
Nectar and pollen |
| 10 |
Coelixys sp. |
Hymenoptera |
Megachilidae |
+ |
+ |
+ |
Nectar and pollen |
| 11 |
Dodona durga |
Lepidoptera |
Riodinidae |
+ |
+ |
+ |
Nectar |
| 12 |
Eupeodes luniger |
Diptera |
Syrphidae |
+ |
- |
+ |
Nectar |
| 13 |
Formica fusca |
Hymenoptera |
Formicidae |
+ |
+ |
+ |
Pollen |
| 14 |
Heliophorus sena |
Lepidoptera |
Lycaenidae |
+ |
- |
+ |
Nectar |
| 15 |
Musa domestica |
Dipteria |
Muscidae |
+ |
+ |
+ |
Nectar |
| 16 |
Plecia sp. |
Dipteria |
Bibionidae |
+ |
- |
- |
Pollen |
| 17 |
Praezygaena caschmirensis |
Lepidoptera |
Zygaenidae |
- |
+ |
- |
Pollen |
| 18 |
Sarcopha sp. |
Diptera |
Sarcophagidae |
+ |
- |
- |
Pollen |
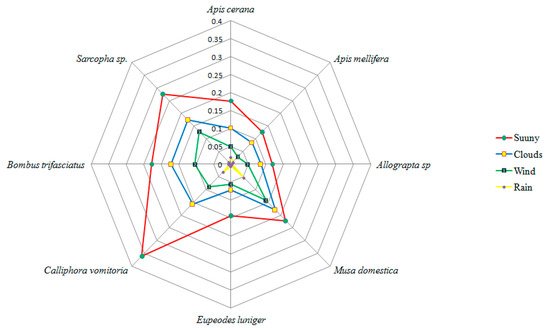
On the basis of insect visiting efficiency indices, Calliphora vomitoria, Bombus trifasciatus, Sarcopha sp., Apis cerana, Apis mellifera, Musa domestica, Apis mellifera, Eupeodes luinger, seem to be the dominant pollinators while Dodona durga, Heliophorus sena, Formica fusca, Coelixys sp., Praezygaena caschmirensis were designated as infrequent visitors. Comparative indices of different insect visitors are provided in Table 4. On normal sunny days with greater illumination and suitable weather conditions, insect foraging starts early in the morning. Highest visitation of the insects was seen between 12:00 and 15:00h which declined thereafter. External environmental factors such as wind, clouds, rain and temperature showed a significant influence on the visiting behavior of insect pollinators. The highest insect visiting efficiency of pollinators was reported on bright sunny days, which declined because of clouds, wind and rain (Figure 4).
Figure 4. Effect of different environmental factors on insect visiting efficiency (measured following the methodology of Yaqoob and Nawchoo
[19]).
Table 4. Comparison for different pollination indices of different insect visitors of
Berberis lycium (measured following the methodology of Yaqoob and Nawchoo
[19]).
| Pollinator |
Foraging Behavior |
Insect Visiting Efficiency |
Foraging Speed |
Index of Visitation Rate |
| Allograpta sp. |
4.1 ± 0.84 |
0.119 ± 0.09 |
20.8 ± 5.5 |
112 ± 23.3 |
| Apis cerana |
6.3 ± 1.4 |
0.176 ± 0.11 |
31 ± 8.2 |
214 ± 27.7 |
| Apis mellifera |
5.91 ± 0.82 |
0.127 ± 0.08 |
27 ± 8.5 |
227 ± 31.1 |
| Bombus trifasciatus |
5.17 ± 2.2 |
0.227 ± 0.09 |
19 ± 6.6 |
204 ± 22.72 |
| Calliphora vomitoria |
9.17 ± 2.19 |
0.361 ± 0.163 |
59.8 ± 16.6 |
389 ± 49.9 |
| Coelixys sp. |
1.59 ± 0.12 |
0.05 ± 0.01 |
6.98 ± 2.21 |
36.06 ± 7.72 |
| Dodona durga |
1.17 ± 0.39 |
0.09 ± 0.068 |
3.03 ± 0.97 |
7.71 ± 2.2 |
| Eupeodes luniger |
3.61 ± 1.19 |
0.143 ± 0.11 |
11.03 ± 2.19 |
47.07 ± 12.02 |
| Formica fusca |
1.23 ± 0.29 |
0.03 ± 0.009 |
5.72 ± 1.9 |
10.17 ± 3.3 |
| Heliophorus sena |
1.03 ± 0.29 |
0.07 ± 0.052 |
2.71 ± 0.73 |
5.51 ± 1.9 |
| Musa domestica |
8.17 ± 0.87 |
0.221 ± 0.12 |
49 ± 11.9 |
271 ± 33.9 |
| Praezygaena caschmirensis |
0.55 ± 0.21 |
0.073 ± 0.021 |
4 ± 1.7 |
6.6 ± 2.54 |
| Sarcopha sp. |
8.09 ± 1.95 |
0.276 ± 0.128 |
51.1 ± 13.3 |
313 ± 36.6 |
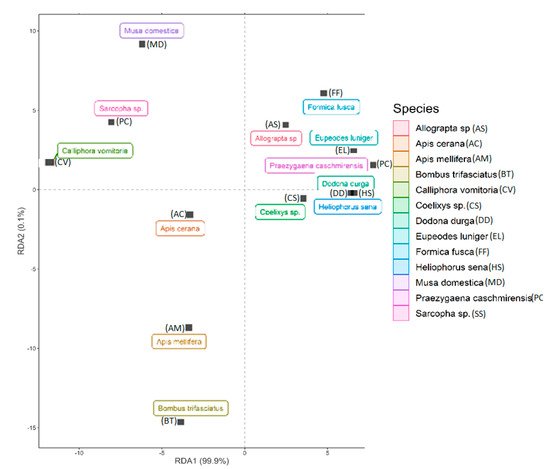
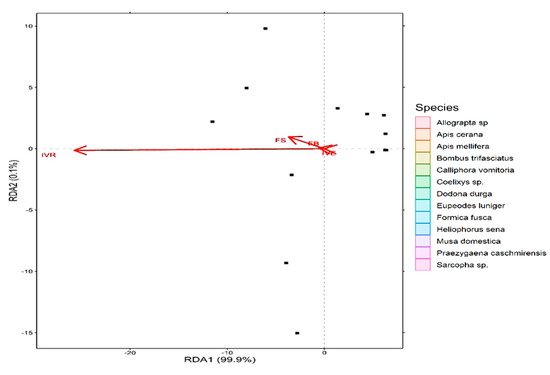
The redundancy analysis (RDA) based on different pollination indices classified different insect visitors into several groups. Apis sp., Calliphora vomitoria, Musa domestica, Sarcopha sp. consists of single isolated species while the rest of the pollinator species were clustered together into same group and show varying degrees of overlap. Further, the first axis (RDA 1) explained about 99.9% of variation in the dataset while the second axis (RDA 2) explained about 0.1% of the variation (Figure 5). Additionally, all the studied pollination indices revealed greater than 40% variation in the data with the highest being reported for index of visitation rate (r = 1), foraging speed (r = 0.99), foraging behavior (r = 0. 96) and insect visiting efficiency (r = 0.85) (Figure 6).
Figure 5. Redundancy analysis (RDA) plot of the studied pollinators based on different pollination indices.
Figure 6. Redundancy analysis (RDA) biplot showing the relationship between studied plant pollinators and the pollination indices contributing greater than 40% variation in the data.