
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ketan Thakare | + 1603 word(s) | 1603 | 2021-08-26 10:08:08 | | | |
| 2 | Amina Yu | + 89 word(s) | 1692 | 2021-09-08 03:29:07 | | |
Video Upload Options
Bioprinting involves the spatial patterning of living cells and other biologics by stacking them using a computer-aided layer-by-layer deposition approach to fabricate living tissue-like constructs. It has the ability to create channels that have features with complex design and is a one-step fabrication process. In addition, it has the potential to be fully automated, maintain accuracy, and be replicated with relative ease.
1. Introduction
The development of new drugs usually takes place in phases over an extended period of time and is an expensive process [1]. A typical drug development process involves four phases before FDA review and approval [2]. In the first phase, drugs are tested in vitro, i.e., the testing occurs using cells or biological materials outside of a living animal. If initial testing is successful, the drug is then investigated in vivo, i.e., using living animal models. However, approximately half of the drugs that pass the first phase fail in later phases [3]. One cause for this high failure rate is that in vitro models are not always able to accurately represent interactions between the drug and the biological environment [4]. Another cause is that standard in vivo animal models often misrepresent the human physiology.
An organ-on-chip system is a microfabricated multichannel 3D microfluidic structure that emulates specific functions of human organs [2][3][4]. Increased specificity of organ-on-chip systems is accomplished by using dynamic fluid flow to provide nutrition and oxygenation with tissue-specific environmental cues and molecular gradients [4][5][6][7][8]. It is foreseen that use of such sophisticated organ-on-chip systems for modeling the activities, mechanics, and physiological responses of human tissues and organs will allow for inexpensive and faster testing of new therapeutic drugs compared with use of traditional in vitro and in vivo animal models [9].
Micro-fabrication methods, including soft-lithography and photolithography, are traditionally used to fabricate organ-on-chip systems [10][11]. In soft lithography, an elastomeric stamp with patterned relief structures on its surface is used to generate patterns and structures (also known as a mold) with feature sizes ranging from 30 nm to 100 µm. Polydimethylsiloxane (PDMS) is then poured into the mold to create a closed-circuit channel sealed with glass slide [10]. In photolithography, a silicon wafer is covered by photoresist material, and ultraviolet (UV) light removes the photoresist material from some portions of the wafer surface. Then, some silicon is etched away from the portions of the wafer not covered with the photoresist material, to create a mold. PDMS is then poured into this mold, to create a closed-circuit channel sealed with a glass slide [11]. However, these methods are expensive and time-consuming [7]. In addition, these methods suffer from limited availability of compatible biological materials [12].
Bioprinting involves the spatial patterning of living cells and other biologics by stacking them using a computer-aided layer-by-layer deposition approach to fabricate living tissue-like constructs [13]. It has the ability to create channels that have features with complex design and is a one-step fabrication process. In addition, it has the potential to be fully automated, maintain accuracy, and be replicated with relative ease [14]. In recent years, bioprinting has been used to produce organ-on-chip systems [15][16].
2. Bioprinting Techniques Used to Fabricate Organ-on-Chip Systems
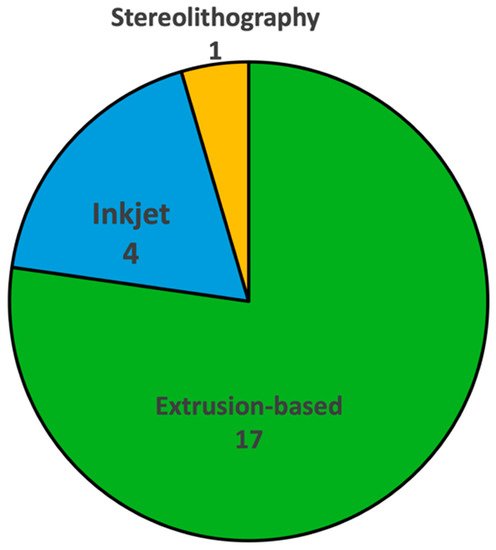
Figure 1 shows the number of reported studies utilizing different bioprinting techniques for fabricating organ-on-chip systems. Out of twenty-two reviewed studies, sixteen used extrusion-based bioprinting, four used inkjet bioprinting, and only one used stereolithography bioprinting. There were no reported studies using laser-based bioprinting. Understanding the working principles of these techniques, along with their advantages and limitations in fabricating organ-on-chip systems, can provide guidance when researchers select bioprinting techniques for organ-on-chip systems. Figure 2 shows schematic illustrations of the three bioprinting techniques that have been used in organ-on-chip fabrication.
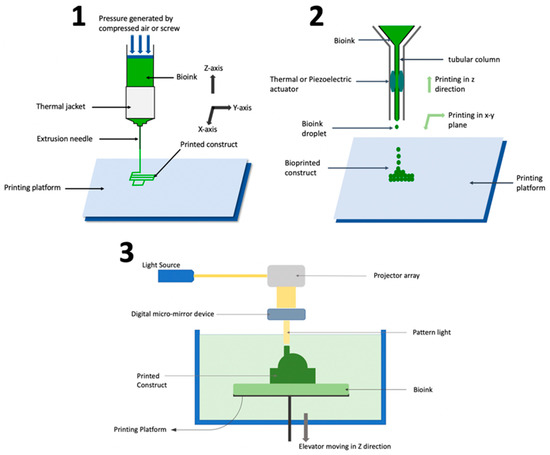
The extrusion-based process is capable of printing a wide array of biomaterials, including composite bioinks that are comparable to natural tissue [17], and therefore is suitable for fabricating organ-on-chip systems that involve several different types of tissues and extracellular matrix components [18]. In addition, the extrusion-based process usually has higher printing speed than other bioprinting processes [13]. Furthermore, the relative simplicity of the technology enables ease-of-use for researchers across disciplines [13]. However, the extrusion-based process has drawbacks such as limited resolution [19], nozzle clogging [20][21], and lower cell viability [22]. Extrusion-based bioprinting process parameters need to be optimized to balance shape fidelity and cell viability. Smaller needle diameters increase the shape fidelity of printed constructs [23] but are harmful to cells due to increased shear stress [24][25]. Increased extrusion pressure is required to print bioinks of high viscosity [20], but this also harms cells through increased shear stress [24][25]. Different cell types have different sensitivities to shear stress [26][27], so the optimization of process parameters depends on the biomaterials used during printing.
In inkjet bioprinting, droplets of bioink are formed through vaporization and dispensed (through an extruder controlled by an actuator) onto a platform in a layer-by-layer fashion to fabricate a 3D construct [28][29][30]. The actuator can have thermal [31] or piezo-electric [32][33] modality for actuation. The extruder can move in the x-y-z directions for fabrication as per the CAD design. However, in certain printers, the extruder is fixed while the printing platform moves in the x-y-z directions.
In inkjet printing, the main printing parameter that can be controlled is droplet size, which is governed by the actuator’s modality. Inkjet bioprinting offers a high resolution (~30 µm) which makes it suitable for fabricating organ-on-chip systems which are in sizes of hundred microns or less [34][35]. In addition, constructs printed with inkjet bioprinting offer high cell viability [34][36]. However, inkjet bioprinting is only suitable for bioinks with low viscosity (~0.1 Pa·s) [37], and the shape fidelity of vertical constructs are poorer with inkjet printing than other bioprinting techniques [34]. Because of these factors, the application of inkjet printing is limited in fabricating organ-on-chip systems that contain tissue types and biomaterials of higher viscosity.
3. Bioink Used in Bioprinting of Organ-on-Chip Systems
In the bioprinting process, from an organ-on-chip fabrication perspective, the bioink formulation needs to satisfy certain physical and biological requirements [38]. The primary biological requirement is that the bioink needs to be biocompatible, which means that it should not be toxic to the cells and should not alter the functionality or physiology of the cells [28]. The second biological requirement is biomimicry, i.e., bioink should mimic the extracellular matrix so that cells can proliferate [39]. Physical properties that the bioink should include are shear-thinning behavior, defined as a non-linear increase in viscosity as stress is applied, and structural fidelity. Shear-thinning behavior is necessary for the bioink to be printable [40]. Bioink also needs to have suitable mechanical properties so that the printed construct is stable [41].
Unique properties of hydrogels make them ideal candidates [42] as bioink constituents. Hydrogels are three dimensional molecules composed of hydrophilic chains which are formed by cross-linking of polymer chains in an aqueous medium through various mechanisms such as physical crosslinking, chemical crosslinking, and photo crosslinking [43]. Crosslinking mechanisms have distinct advantages and disadvantages. Photo crosslinked constructs have high shape fidelity, but the UV light used during photo crosslinking can create free radicals which are harmful to cells [44]. Ionic crosslinking, a common form of physical crosslinking, forms mechanically weaker constructs than chemical crosslinking, but promotes higher cell viability [45]. Hydrogels have the ability to absorb water up to a thousand times their dry weight, which makes them suitable materials to act as extracellular matrix and support cell proliferation. The reviewed studies have used natural hydrogels like alginate, gelatin, cellulose, fibrin, and collagen, and synthetic hydrogels such as poly (ethylene glycol) (PEG), poly (ɛ-caprolactone) (PCL), pluronic, and gelatin methacryloyl (GelMA) as shown in Table 1 .
| Hydrogel as Bioink Constituent | Bioprinting Technique | Crosslinking Mechanism | Organ-on-Chip System | Reference |
|---|---|---|---|---|
| Alginate | Extrusion | Physical | Vessel, heart | [46][47] |
| Gelatin | Extrusion, stereolithography | Chemical | Vessel, liver, kidney | [48][49][50][51] |
| Cellulose | Extrusion | Chemical | Tumor | [52] |
| Fibrin | Extrusion, inkjet | Physical | Vessel, kidney | [49][50][53] |
| Collagen | Extrusion, inkjet | Chemical | Vessel, gut, lung | [51][53][54][55] |
| Poly (ethylene glycol) (PEG) | Stereolithography | Photo | Liver | [48] |
| Poly (ε-caprolactone) (PCL) | Extrusion | Photo | Liver | [56] |
| Gelatin methacryloyl (GelMA) | Extrusion, inkjet | Photo | Vessel, heart, liver, tumor | [47][57][58][59][60] |
| Pluronic | Extrusion | Photo | Kidney | [49] |
4. Organ-on-Chip Systems Fabricated Using Bioprinting
In a similar study, Abudupataer et al. used extrusion-based bioprinting to fabricate a vessel-on-chip system [57]. Two layers of bioink containing endothelial cells and muscle cells were printed on chip (fabricated with PDMA), and after the cells proliferated, a continuous flow of growth medium was perfused in the channel of the chip to mimic blood flow in the vessel. Such a vessel-on-chip model can be used to study the pathogenesis of disease and drug screening.
Cancer is an umbrella term for a variety of diseases having the same underlying cause of unregulated division of cells, which can be a cause of morbidity and mortality. Cancer treatment is especially challenging due to different tumor characteristics (mass of tissue caused by unregulated division of cells) in different patients. Tumor-on-chip systems tackle this heterogeneity by enabling the development of patient-specific anti-cancer drugs targeted to treat the patient-specific tumor.
Mi et al. used inkjet bioprinting to fabricate a breast tumor-on-chip system [61]. Breast cancer cells and endothelial cells were printed on a PDMS chip (fabricated with soft-lithography). Post printing, the cells showed good cell viability and cell quality. A significant inhibition of tumor cell migration ability was observed when treated with paclitaxel (anti-cancer drug), demonstrating the effectiveness of such a tumor-on-chip system in aiding cancer research and for anti-cancer drug screening. Successful cell proliferation and cell integration in co-culture showed that such a co-culture tumors-on-chip system is viable for biological characterization.
Cheng et al. used extrusion-based bioprinting to print paper-based cancer tissue models which have the potential for application in the cost-effective fabrication of organ-on-chip models [52]. In this study, sacrificial petroleum jelly-liquid paraffin ink was used to print on bacterial cellulose hydrogel, the entire matrix was air-dried to form a paper-like membrane, and perfusable microchannels were obtained by removing the sacrificial ink using heat. The epithelial cells were seeded into the microchannels and cancer cells were seeded onto the surface of the paper-based device. It was observed that endothelial cells and tumor cells spread and proliferated, and cytotoxicity of cancer cells was observed on treatment with tamoxifen (anti-cancer drug), demonstrating that such paper-based models can possibly be used for producing tumor-on-chip systems for drug screening.
References
- Morgan, S.; Grootendorst, P.; Lexchin, J.; Cunningham, C.; Greyson, D. The cost of drug development: A systematic review. Health Policy 2011, 100, 4–17.
- Lipsky, M.S.; Sharp, L.K. From idea to market: The drug approval process. J. Am. Board Fam. Pract. 2001, 14, 362–367.
- Staff, Reuters. U.S. to develop chip that tests if a drug is toxic. Reuters, 16 September 2011.
- Ghaemmaghami, A.M.; Hancock, M.J.; Harrington, H.; Kaji, H.; Khademhosseini, A. Biomimetic tissues on a chip for drug discovery. Drug Discov. Today 2012, 17, 173–181.
- Elçin, Y.M. Organs-on-Chips & 3D—Bioprinting Technologies for Personalized Medicine. Stem Cell Rev. Rep. 2017, 13, 319–320.
- Leary, J.F.; Key, J.; Vidi, P.-A.; Cooper, C.L.; Kole, A.; Reece, L.M.; Lelièvre, S.A. Human organ-on-a-chip BioMEMS devices for testing new diagnostic and therapeutic strategies. In Proceedings of the Microfluidics, BioMEMS, and Medical Microsystems XI, International Society for Optics and Photonics, San Francisco, CA, USA, 3–6 February 2013; p. 86150A.
- Yang, Q.; Lian, Q.; Xu, F. Perspective: Fabrication of integrated organ-on-a-chip via bioprinting. Biomicrofluidics 2017, 11, 031301.
- Moyer, M.W. Organs-on-a-Chip for Faster Drug Development. In Scientific American; Springer Nature: Basingstoke, UK, 2011.
- Avci, H.; Güzel, F.D.; Erol, S.; Akpek, A. Recent advances in organ-on-a-chip technologies and future challenges: A review. Turk. J. Chem. 2018, 42, 587–610.
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184.
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772.
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286.
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343.
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.-J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637.
- Au, A.K.; Lee, W.; Folch, A. Mail-order microfluidics: Evaluation of stereolithography for the production of microfluidic devices. Lab Chip 2014, 14, 1294–1301.
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742.
- Chen, S.; Jang, T.S.; Pan, H.M.; Jung, H.D.; Sia, M.W.; Xie, S.; Hang, Y.; Chong, S.; Wang, D.; Song, J. 3D freeform printing of nanocomposite hydrogels through in situ precipitation in reactive viscous fluid. Int. J. Bioprint. 2020, 6, 258.
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 1–11.
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264.
- Ning, L.; Chen, X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017, 12, 1600671.
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161.
- Tanzeglock, T.; Soos, M.; Stephanopoulos, G.; Morbidelli, M. Induction of mammalian cell death by simple shear and extensional flows. Biotechnol. Bioeng. 2009, 104, 360–370.
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022.
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107.
- Cidonio, G.; Glinka, M.; Dawson, J.I.; Oreffo, R.O.C. The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine. Biomaterials 2019, 209, 10–24.
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218.
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the field of 3D biomaterial printing. Biomed. Mater. 2016, 11, 014102.
- Ji, S.; Guvendiren, M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front. Bioeng. Biotechnol. 2017, 5, 23.
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080.
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A review of three-dimensional printing in tissue engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310.
- Kador, K.E.; Grogan, S.P.; Dorthé, E.W.; Venugopalan, P.; Malek, M.F.; Goldberg, J.L.; D’lima, D.D. Control of retinal ganglion cell positioning and neurite growth: Combining 3D printing with radial electrospun scaffolds. Tissue Eng. Part A 2016, 22, 286–294.
- Cheng, E.; Yu, H.; Ahmadi, A.; Cheung, K.C. Investigation of the hydrodynamic response of cells in drop on demand piezoelectric inkjet nozzles. Biofabrication 2016, 8, 015008.
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055.
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434.
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent US4575330A, 11 March 1986.
- Wilson, W.C., Jr.; Boland, T. Cell and organ printing 1: Protein and cell printers. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 272, 491–496.
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305.
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239.
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785.
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272.
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055.
- Gruene, M.; Unger, C.; Koch, L.; Deiwick, A.; Chichkov, B. Dispensing pico to nanolitre of a natural hydrogel by laser-assisted bioprinting. Biomed. Eng. Online 2011, 10, 19.
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479.
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.; Burdick, J.A.; Woodfield, T.B. Fundamentals and applications of photo-cross-linking in bioprinting. Chem. Rev. 2020, 120, 10662–10694.
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small 2020, 16, 2002931.
- Gao, Q.; Liu, Z.; Lin, Z.; Qiu, J.; Liu, Y.; Liu, A.; Wang, Y.; Xiang, M.; Chen, B.; Fu, J. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater. Sci. Eng. 2017, 3, 399–408.
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59.
- Grix, T.; Ruppelt, A.; Thomas, A.; Amler, A.-K.; Noichl, B.P.; Lauster, R.; Kloke, L. Bioprinting perfusion-enabled liver equivalents for advanced organ-on-a-chip applications. Genes 2018, 9, 176.
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep. 2016, 6, 34845.
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184.
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.-S.; Vincent, P.A.; Dai, G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35, 8092–8102.
- Cheng, F.; Cao, X.; Li, H.; Liu, T.; Xie, X.; Huang, D.; Maharjan, S.; Bei, H.P.; Gómez, A.; Li, J. Generation of cost-effective paper-based tissue models through matrix-assisted sacrificial 3D printing. Nano Lett. 2019, 19, 3603–3611.
- Schöneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.J.; Kießling, F.; Fischer, H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 2018, 8, 1–13.
- Kim, W.; Kim, G. Intestinal villi model with blood capillaries fabricated using collagen-based bioink and dual-cell-printing process. ACS Appl. Mater. Interfaces 2018, 10, 41185–41196.
- Park, J.Y.; Ryu, H.; Lee, B.; Ha, D.-H.; Ahn, M.; Kim, S.; Kim, J.Y.; Jeon, N.L.; Cho, D.-W. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 2018, 11, 015002.
- Lee, H.; Cho, D.-W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016, 16, 2618–2625.
- Abudupataer, M.; Chen, N.; Yan, S.; Alam, F.; Shi, Y.; Wang, L.; Lai, H.; Li, J.; Zhu, K.; Wang, C. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomed. Microdevices 2020, 22, 10.
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101.
- Cao, X.; Ashfaq, R.; Cheng, F.; Maharjan, S.; Li, J.; Ying, G.; Hassan, S.; Xiao, H.; Yue, K.; Zhang, Y.S. A tumor-on-a-chip system with bioprinted blood and lymphatic vessel pair. Adv. Funct. Mater. 2019, 29, 1807173.
- Zhang, Y.S.; Davoudi, F.; Walch, P.; Manbachi, A.; Luo, X.; Dell’Erba, V.; Miri, A.K.; Albadawi, H.; Arneri, A.; Li, X. Bioprinted thrombosis-on-a-chip. Lab Chip 2016, 16, 4097–4105.
- Mi, S.; Yang, S.; Liu, T.; Du, Z.; Xu, Y.; Li, B.; Sun, W. A novel controllable cell array printing technique on microfluidic chips. IEEE Trans. Biomed. Eng. 2019, 66, 2512–2520.




