
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alazne Moreno Lanceta | + 4991 word(s) | 4991 | 2021-08-30 09:03:58 | | | |
| 2 | Beatrix Zheng | Meta information modification | 4991 | 2021-09-02 12:40:28 | | |
Video Upload Options
Nanoparticles are nanomaterials with three external nanoscale dimensions and an average size ranging from 1 to 1000 nm. Nanoparticles have gained notoriety in technological advances due to their tunable physical, chemical, and biological characteristics. However, the administration of functionalized nanoparticles to living beings is still challenging due to the rapid detection and blood and tissue clearance by the mononuclear phagocytic system. The major exponent of this system is the macrophage. Regardless the nanomaterial composition, macrophages can detect and incorporate foreign bodies by phagocytosis. Therefore, the simplest explanation is that any injected nanoparticle will be probably taken up by macrophages. This explains, in part, the natural accumulation of most nanoparticles in the spleen, lymph nodes, and liver (the main organs of the mononuclear phagocytic system). For this reason, recent investigations are devoted to design nanoparticles for specific macrophage targeting in diseased tissues. The aim of this review is to describe current strategies for the design of nanoparticles to target macrophages and to modulate their immunological function involved in different diseases with special emphasis on chronic inflammation, tissue regeneration, and cancer.
1. Introduction
Macrophages are plastic cells from the innate immune system that play different roles in the development, homeostasis, tissue repair, and immune response [1]. The local tissue microenvironment determines the macrophage polarization phenotype, M1-like or M2-like, in such a way that populations of both subsets can be found simultaneously coexisting in the same tissue. Macrophages are classically activated into the pro-inflammatory M1 phenotype in response to inflammatory stimuli such as lipopolysaccharides (LPS) or interferon-γ (IFN-γ). On the other hand, interleukin-4 (IL-4) and IL-13 alternatively activate the macrophage polarization to an anti-inflammatory M2 phenotype [2]. Polarized macrophages can be also reprogrammed by the combination of different agents promoting a phenotype reversion [3].
Damaged cells release specific molecules known as damage-associated molecular patterns (DAMPs) that activate the immune system in an analogous manner to pathogen-associated molecular patterns (PAMPs), small molecular motifs released from bacteria or viruses [4]. These endogenous molecules display physiological functions in cells, but are recognized as danger signals when released into the extracellular space leading to downstream inflammation. Therefore, tissue-specific macrophage subpopulations detect signals that are not found in healthy tissues following infection or injury and recruit monocytes that differentiate into macrophages [2]. DAMPs, PAMPS, or IFN-γ secreted by lymphocytes induce the pro-inflammatory M1 macrophage phenotype [3]. M1 macrophages secrete a variety of pro-inflammatory mediators such as IL-1 and tumour necrosis factor (TNFα) that stimulate inflammation, and IL-12, which activates T helpers 1 (TH1), initiating the adaptive immune response [2]. M1 macrophages also secrete reactive oxygen species (ROS) and nitrogen species that contribute to the elimination of invading organisms. During this process, they also trigger substantial collateral tissue damage to the host. To prevent further tissue damage due to the inflammatory macrophage response, macrophages undergo apoptosis or polarization to an anti-inflammatory and pro-regenerative phenotype that dampens the pro-inflammatory response and facilitates wound healing [1]. IL-4 and IL-13 alternatively activate the macrophage polarization to an anti-inflammatory M2 phenotype [2]. M2 macrophages secrete anti-inflammatory cytokines such as IL-4, IL-13, or IL-10 to dampen the proinflammatory response [2] and specific and numerous growth factors such as transforming growth factor (TGFβ1) and vascular endothelial growth factors (VEGFs) to promote cell proliferation and angiogenesis [5]. M2 macrophages can also regulate the proliferation and expansion of neighboring parenchymal and stromal cells and the activation of stem cells and local progenitor cell populations that participate in repair [5].
The inflammatory and anti-inflammatory responses orchestrated by macrophages need to be accurately regulated to prevent disease. Cytokines are the signals that mediate the coordination between immune cells to harmonize the balance between inflammation and tissue repair [6]. Imbalance of M1/M2 macrophage populations is associated with different diseases [3]. Uncontrolled inflammatory response driven by macrophages leads to chronic inflammation and autoimmune diseases [1]. Similarly, the dysregulation of anti-inflammatory response can contribute to tumour progression and metastasis [7]. In addition, prolonged inflammation and continuous activation of macrophages results in chronic diseases that may lead to the development of pathological fibrosis. In some diseases, extensive fibrosis can ultimately lead to organ failure and death [5].
Different types of biomaterials such as nanoparticles (NPs) and hydrogels are being extensively developed to target macrophages. Since macrophages are professional phagocytic cells, NPs can be exploited as vehicles that naturally target macrophages. These immune cells can easily incorporate NPs via phagocytosis, macropinocytosis, or receptor-mediated endocytosis [8]. Some types of NPs can interact with macrophages to directly modify their biological functions [6]. In addition, NPs can be used as drug delivery systems to treat macrophages involved in different diseases [9]. Several therapeutic options using functionalized NPs are being explored and developed to modulate macrophages.
This review summarizes the use of NPs to modulate macrophages involved in the initiation and progress of different diseases. We will particularly focus on NPs to target and treat macrophages involved in diseases characterized by chronic inflammation and in tumor-associated macrophages (TAMs).
2. Nanoparticles to Modulate Macrophages in Chronic Inflammation
The persistence of the harmful agent and the propagation of the inflammatory response leads to the imbalance between inflammatory and anti-inflammatory signals. In this scenario, macrophages are potential targets to treat inflammatory disorders such as rheumatoid arthritis, atherosclerosis, and inflammatory bowel disease. Drug-loaded NPs can be used to modulate the immunological activity of the pro-inflammatory M1 macrophages or to switch their phenotype from M1 to M2 [10][11]. These therapeutic strategies are designed to shift the dynamic balance from pro- to anti-inflammatory signals to mitigate the pathological process [3].
2.1. NPs Modulating Macrophages in Rheumatoid Arthritis
2.2. NPs Modulating Macrophages in Inflammatory Bowel Disease (IBD)
Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal (GI) tract. There are two types of IBD: Crohn disease (CD) and ulcerative colitis (UC) [6]. Macrophages are an important source of proinflammatory cytokines (such as TNFα) that play an important role on the pathogenesis of IBD. TNFα has become an attractive target for IBD therapy using NPs. Most studies have investigated the use of different NPs containing TNFα siRNA as an interesting therapeutic strategy. Xiao and colleagues have used mannosylated NPs as delivery vehicles for TNFα siRNA [21]. TNFα siRNA demonstrated an effective activity to drastically reduce the TNFα expression and promoted anti-inflammatory effects in vitro and ex vivo leading to colitis attenuation in a dextran sodium sulfate (DSS)-induced colitis mouse model [21]. Similar results were obtained by H. Laroui and colleagues. They designed NPs with a Fab’ portion of the F4/80 antibody against murine macrophages, which also contained TNFα-siRNA showing high efficiency in the attenuation of colitis [22]. Wilson and colleagues have also encapsulated TNFα siRNA in NPs, but they have designed stimulus-responsive NPs. They have developed thioketal NPs (TKNs) that are reactive to high concentration of ROS [23]. Other strategies have combined the delivery of TNFα siRNA with siRNA against Cyclin D1 [24]. Kriegel and Amiji have used NPs in microsphere oral systems for dual siRNA (TNFα and Cyclin D1) delivery [24]. This dual treatment has shown to be more effective than each agent separately for treating IBD in a DSS-induced mouse model.
2.3. NPs Modulating Macrophages in Atherosclerosis
3. Nanoparticles to Stimulate Tissue Repair and Regeneration
M2 macrophages are actively involved in tissue repair and wound healing. For this reason, many investigations nowadays are dedicated to design NPs to stimulate the M2 macrophage phenotype for tissue or organ regeneration [31].
3.1. NPs Modulating Macrophages in Myocardial Infarct Repair
Myocardial infarction (MI) is the result of partial or complete coronary artery occlusion that leads to blood flow reduction [6][32]. The appropriate myocardial healing is guided by macrophages [26]. For this reason, numerous NPs have been developed to target macrophages and achieve myocardial infarct repair.
3.2. NPs Modulating Macrophages in Chronic Liver Injury
4. Nanoparticles to Target and Treat Tumour-Associated Macrophages
4.1. Specific Differential Phenotype of Tumour-Associated Macrophages
4.2. NPs to Target TAM for Cancer Diagnostics and Prognosis
4.3. NPs to Inhibit Macrophage Recruitment and to Deplete TAM in Tumors
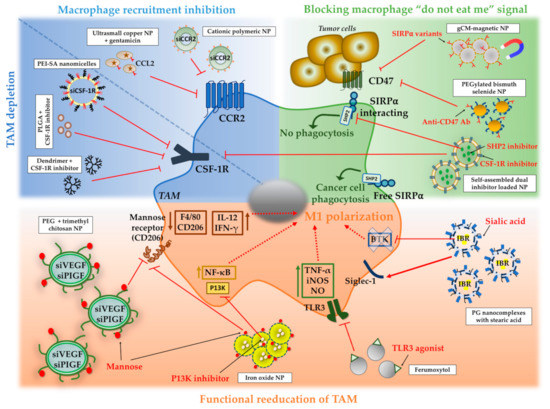
4.4. NPs to Block the Macrophage “Do Not Eat Me” Signal
4.5. NPs to Switch TAM to an Antitumor “M1-Like” Phenotype
5. Nanocomposite Hydrogels to Modulate Macrophages
References
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455.
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737.
- Miao, X.; Leng, X.; Zhang, Q. The current state of nanoparticle-induced macrophage polarization and reprogramming research. Int. J. Mol. Sci. 2017, 18, 336.
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289.
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462.
- Nakkala, J.R.; Li, Z.; Ahmad, W.; Wang, K.; Gao, C. Immunomodulatory biomaterials and their application in therapies for chronic inflammation-related diseases. Acta Biomater. 2021, 123, 1–30.
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16.
- Garash, R.; Bajpai, A.; Marcinkiewicz, B.M.; Spiller, K.L. Drug delivery strategies to control macrophages for tissue repair and regeneration. Exp. Biol. Med. 2016, 241, 1054–1063.
- Ponzoni, M.; Pastorino, F.; Di Paolo, D.; Perri, P.; Brignole, C. Targeting macrophages as a potential therapeutic intervention: Impact on inflammatory diseases and cancer. Int. J. Mol. Sci. 2018, 19, 1953.
- Hu, G.; Guo, M.; Xu, J.; Wu, F.; Fan, J.; Huang, Q.; Yang, G.; Lv, Z.; Wang, X.; Jin, Y. Nanoparticles targeting macrophages as potential clinical therapeutic agents against cancer and inflammation. Front. Immunol. 2019, 10, 1998.
- Alvarez, M.M.; Liu, J.C.; Trujillo-de Santiago, G.; Cha, B.H.; Vishwakarma, A.; Ghaemmaghami, A.M.; Khademhosseini, A. Delivery strategies to control inflammatory response: Modulating M1–M2 polarization in tissue engineering applications. J. Control Release 2016, 240, 349–363.
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15.
- Jain, S.; Tran, T.H.; Amiji, M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials 2015, 61, 162–177.
- Singh, A.; Talekar, M.; Raikar, A.; Amiji, M. Macrophage-targeted delivery systems for nucleic acid therapy of inflammatory diseases. J. Control Release 2014, 190, 515–530.
- Howard, K.A.; Paludan, S.R.; Behlke, M.A.; Besenbacher, F.; Deleuran, B.; Kjems, J. Chitosan/siRNA nanoparticle-mediated TNF-α knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol. Ther. 2009, 17, 162–168.
- Fernandes, J.C.; Wang, H.; Jreyssaty, C.; Benderdour, M.; Lavigne, P.; Qiu, X.; Winnik, F.M.; Zhang, X.; Dai, K.; Shi, Q. Bone-protective effects of nonviral gene therapy with folate-chitosan DNA nanoparticle containing interleukin-1 receptor antagonist gene in rats with adjuvant-induced arthritis. Mol. Ther. 2008, 16, 1243–1251.
- Xiao, S.; Tang, Y.; Lv, Z.; Lin, Y.; Chen, L. Nanomedicine—Advantages for their use in rheumatoid arthritis theranostics. J. Control Release 2019, 316, 302–316.
- Van Der Heijden, J.W.; Oerlemans, R.; Dijkmans, B.A.C.; Qi, H.; Van Der Laken, C.J.; Lems, W.F.; Jackman, A.L.; Kraan, M.C.; Tak, P.P.; Ratnam, M.; et al. Folate receptor β as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis Rheumatol. 2009, 60, 12–21.
- Thomas, T.P.; Goonewardena, S.N.; Majoros, I.J.; Kotlyar, A.; Cao, Z.; Leroueil, P.R.; Baker, J.R. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheumatol. 2011, 63, 2671–2680.
- Zhao, J.; Zhao, M.; Yu, C.; Zhang, X.; Liu, J.; Cheng, X.; Lee, R.J.; Sun, F.; Teng, L.; Li, Y. Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis. Int. J. Nanomed. 2017, 12, 6735–6746.
- Xiao, B.; Laroui, H.; Ayyadurai, S.; Viennois, E.; Charania, M.A.; Zhang, Y.; Merlin, D. Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy. Biomaterials 2013, 34, 7471–7482.
- Laroui, H.; Viennois, E.; Xiao, B.; Canup, B.S.B.; Geem, D.; Denning, T.L.; Merlin, D. Fab’-bearing siRNA TNFα-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J. Control Release 2014, 186, 41–53.
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928.
- Kriegel, C.; Amiji, M.M. Dual TNF-α/Cyclin D1 gene silencing with an oral polymeric microparticle system as a novel strategy for the treatment of inflammatory bowel disease. Clin. Transl. Gastroenterol. 2011, 2, e2.
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622.
- Zang, X.; Cheng, M.; Zhang, X.; Chen, X. Targeting macrophages using nanoparticles: A potential therapeutic strategy for atherosclerosis. J. Mater. Chem. B 2021, 9, 3284–3294.
- He, H.; Wang, J.; Yannie, P.J.; Korzun, W.J.; Yang, H.; Ghosh, S. Nanoparticle-based “Two-pronged” approach to regress atherosclerosis by simultaneous modulation of cholesterol influx and efflux. Biomaterials 2020, 260, 120333.
- Zhao, Y.; He, Z.; Gao, H.; Tang, H.; He, J.; Guo, Q.; Zhang, W.; Liu, J. Fine Tuning of Core-Shell Structure of Hyaluronic Acid/Cell-Penetrating Peptides/siRNA Nanoparticles for Enhanced Gene Delivery to Macrophages in Antiatherosclerotic Therapy. Biomacromolecules 2018, 19, 2944–2956.
- He, H.; Yuan, Q.; Bie, J.; Wallace, R.L.; Yannie, P.J.; Wang, J.; Lancina, M.G.; Zolotarskaya, O.Y.; Korzun, W.; Yang, H.; et al. Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: Use of this platform to modulate atherosclerosis. Transl. Res. 2018, 193, 13–30.
- Tao, W.; Yurdagul, A.; Kong, N.; Li, W.; Wang, X.; Doran, A.C.; Feng, C.; Wang, J.; Islam, M.A.; Farokhzad, O.C.; et al. SiRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 2020, 12, eaay1063.
- Shen, P.; Chen, Y.; Luo, S.; Fan, Z.; Wang, J.; Chang, J.; Deng, J. Applications of biomaterials for immunosuppression in tissue repair and regeneration. Acta Biomater. 2021, 126, 31–44.
- Bejerano, T.; Etzion, S.; Elyagon, S.; Etzion, Y.; Cohen, S. Nanoparticle Delivery of miRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018, 18, 5885–5891.
- Harel-Adar, T.; Mordechai, T.B.; Amsalem, Y.; Feinberg, M.S.; Leor, J.; Cohen, S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc. Natl. Acad. Sci. USA 2011, 108, 1827–1832.
- Courties, G.; Heidt, T.; Sebas, M.; Iwamoto, Y.; Jeon, D.; Truelove, J.; Tricot, B.; Wojtkiewicz, G.; Dutta, P.; Sager, H.B.; et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J. Am. Coll. Cardiol. 2014, 63, 1556–1566.
- Tokutome, M.; Matoba, T.; Nakano, Y.; Okahara, A.; Fujiwara, M.; Koga, J.I.; Nakano, K.; Tsutsui, H.; Egashira, K. Peroxisome proliferator-activated receptor-gamma targeting nanomedicine promotes cardiac healing after acute myocardial infarction by skewing monocyte/macrophage polarization in preclinical animal models. Cardiovasc. Res. 2019, 115, 419–431.
- Ju, C.; Tacke, F. Hepatic macrophages in homeostasis and liver diseases: From pathogenesis to novel therapeutic strategies. Cell. Mol. Immunol. 2016, 13, 316–327.
- He, C.; Yin, L.; Tang, C.; Yin, C. Multifunctional polymeric nanoparticles for oral delivery of TNF-α siRNA to macrophages. Biomaterials 2013, 34, 2843–2854.
- Kurniawan, D.W.; Jajoriya, A.K.; Dhawan, G.; Mishra, D.; Argemi, J.; Bataller, R.; Storm, G.; Mishra, D.P.; Prakash, J.; Bansal, R. Therapeutic inhibition of spleen tyrosine kinase in inflammatory macrophages using PLGA nanoparticles for the treatment of non-alcoholic steatohepatitis. J. Control Release 2018, 288, 227–238.
- Bartneck, M.; Scheyda, K.M.; Warzecha, K.T.; Rizzo, L.Y.; Hittatiya, K.; Luedde, T.; Storm, G.; Trautwein, C.; Lammers, T.; Tacke, F. Fluorescent cell-traceable dexamethasone-loaded liposomes for the treatment of inflammatory liver diseases. Biomaterials 2015, 37, 367–382.
- Melgar-Lesmes, P.; Luquero, A.; Parra-Robert, M.; Mora, A.; Ribera, J.; Edelman, E.R.; Jiménez, W. Graphene-Dendrimer Nanostars for Targeted Macrophage Overexpression of Metalloproteinase 9 and Hepatic Fibrosis Precision Therapy. Nano Lett. 2018, 18, 5839–5845.
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61.
- Qian, B.Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51.
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L. Europe PMC Funders Group Tumor-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416.
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327.
- Petty, A.J.; Yang, Y. Tumor-associated macrophages: Implications in cancer immunotherapy. Immunotherapy 2017, 9, 289–302.
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631.
- Koebel, C.M.; Vermi, W.; Swann, J.B.; Zerafa, N.; Rodig, S.J.; Old, L.J.; Smyth, M.J.; Schreiber, R.D. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007, 450, 903–907.
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188.
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2021, 6, 1973–1987.
- Leimgruber, A.; Berger, C.; Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.P.; Waterman, P.; Figueiredo, J.L.; Kohler, R.H.; Elpek, N.; Mempel, T.R.; et al. Behavior of endogenous Tumor-associated macrophages assessed in vivo using a functionalized nanoparticle. Neoplasia 2009, 11, 459–468.
- Daldrup-Link, H.E.; Golovko, D.; Ruffell, B.; DeNardo, D.G.; Castaneda, R.; Ansari, C.; Rao, J.; Tikhomirov, G.A.; Wendland, M.F.; Corot, C.; et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin. Cancer Res. 2011, 17, 5695–5704.
- Ng, T.S.C.; Gunda, V.; Li, R.; Prytyskach, M.; Iwamoto, Y.; Kohler, R.H.; Parangi, S.; Weissleder, R.; Miller, M.A. Detecting immune response to therapies targeting PDL1 and BRAF by using ferumoxytol MRI and macrin in anaplastic thyroid cancer. Radiology 2020, 298, 123–132.
- Baroni, S.; Ruggiero, M.R.; Bitonto, V.; Broche, L.M.; Lurie, D.J.; Aime, S.; Geninatti Crich, S. In vivo assessment of tumour associated macrophages in murine melanoma obtained by low-field relaxometry in the presence of iron oxide particles. Biomaterials 2020, 236, 119805.
- Shin, S.H.; Park, S.H.; Kang, S.H.; Kim, S.W.; Kim, M.; Kim, D. Fluorine-19 magnetic resonance imaging and positron emission tomography of tumor-associated macrophages and tumor metabolism. Contrast Media Mol. Imaging 2017, 2017, 4896310.
- Pérez-Medina, C.; Tang, J.; Abdel-Atti, D.; Hogstad, B.; Merad, M.; Fisher, E.A.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.M.; Reiner, T. PET imaging of tumor-associated macrophages with 89Zr-labeled high-density lipoprotein nanoparticles. J. Nucl. Med. 2015, 56, 1272–1277.
- Kim, H.Y.; Li, R.; Ng, T.S.C.; Courties, G.; Rodell, C.B.; Prytyskach, M.; Kohler, R.H.; Pittet, M.J.; Nahrendorf, M.; Weissleder, R.; et al. Quantitative Imaging of Tumor-Associated Macrophages and Their Response to Therapy Using 64 Cu-Labeled Macrin. ACS Nano 2018, 12, 12015–12029.
- Locke, L.W.; Mayo, M.W.; Yoo, A.D.; Williams, M.B.; Berr, S.S. PET imaging of tumor associated macrophages using mannose coated 64Cu liposomes. Biomaterials 2012, 33, 7785–7793.
- Lee, C.; Kim, G.R.; Yoon, J.; Kim, S.E.; Yoo, J.S.; Piao, Y. In vivo delineation of glioblastoma by targeting tumor-associated macrophages with near-infrared fluorescent silica coated iron oxide nanoparticles in orthotopic xenografts for surgical guidance. Sci. Rep. 2018, 8, 11122.
- Shen, S.; Zhang, Y.; Chen, K.G.; Luo, Y.L.; Wang, J. Cationic Polymeric Nanoparticle Delivering CCR2 siRNA to Inflammatory Monocytes for Tumor Microenvironment Modification and Cancer Therapy. Mol. Pharm. 2018, 15, 3642–3653.
- Zhang, X.; Detering, L.; Sultan, D.; Luehmann, H.; Li, L.; Heo, G.S.; Zhang, X.; Lou, L.; Grierson, P.M.; Greco, S.; et al. CC Chemokine Receptor 2-Targeting Copper Nanoparticles for Positron Emission Tomography-Guided Delivery of Gemcitabine for Pancreatic Ductal Adenocarcinoma. ACS Nano 2021, 15, 1186–1198.
- Li, M.; Li, M.; Yang, Y.; Liu, Y.; Xie, H.; Yu, Q.; Tian, L.; Tang, X.; Ren, K.; Li, J.; et al. Remodeling tumor immune microenvironment via targeted blockade of PI3K-γ and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J. Control Release 2020, 321, 23–35.
- Qian, Y.; Qiao, S.; Dai, Y.; Xu, G.; Dai, B.; Lu, L.; Yu, X.; Luo, Q.; Zhang, Z. Molecular-Targeted Immunotherapeutic Strategy for Melanoma via Dual-Targeting Nanoparticles Delivering Small Interfering RNA to Tumor-Associated Macrophages. ACS Nano 2017, 11, 9536–9549.
- Pang, L.; Pei, Y.; Uzunalli, G.; Hyun, H.; Lyle, L.T.; Yeo, Y. Surface Modification of Polymeric Nanoparticles with M2pep Peptide for Drug Delivery to Tumor-Associated Macrophages. Pharm. Res. 2019, 36, 65.
- Liaw, K.; Reddy, R.; Sharma, A.; Li, J.; Chang, M.; Sharma, R.; Salazar, S.; Kannan, S.; Kannan, R.M. Targeted systemic dendrimer delivery of CSF-1R inhibitor to tumor-associated macrophages improves outcomes in orthotopic glioblastoma. Bioeng. Transl. Med. 2021, 6, e10205.
- Wang, Y.; Luan, Z.; Zhao, C.; Bai, C.; Yang, K. Target delivery selective CSF-1R inhibitor to tumor-associated macrophages via erythrocyte-cancer cell hybrid membrane camouflaged pH-responsive copolymer micelle for cancer immunotherapy. Eur. J. Pharm. Sci. 2020, 142, 105136.
- Cuccarese, M.F.; Dubach, J.M.; Pfirschke, C.; Engblom, C.; Garris, C.; Miller, M.A.; Pittet, M.J.; Weissleder, R. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat. Commun. 2017, 8, 14293.
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; van den Berg, T.K. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164.
- Matozaki, T.; Murata, Y.; Okazawa, H.; Ohnishi, H. Functions and molecular mechanisms of the CD47-SIRPα signalling pathway. Trends Cell Biol. 2009, 19, 72–80.
- Rao, L.; Zhao, S.K.; Wen, C.; Tian, R.; Lin, L.; Cai, B.; Sun, Y.; Kang, F.; Yang, Z.; He, L.; et al. Activating Macrophage-Mediated Cancer Immunotherapy by Genetically Edited Nanoparticles. Adv. Mater. 2020, 32, 2004853.
- Guo, Z.; Liu, Y.; Zhou, H.; Zheng, K.; Wang, D.; Jia, M.; Xu, P.; Ma, K.; Cui, C.; Wang, L. CD47-targeted bismuth selenide nanoparticles actualize improved photothermal therapy by increasing macrophage phagocytosis of cancer cells. Colloids Surf. B Biointerfaces 2019, 184, 110546.
- Ramesh, A.; Kumar, S.; Nandi, D.; Kulkarni, A. CSF1R- and SHP2-Inhibitor-Loaded Nanoparticles Enhance Cytotoxic Activity and Phagocytosis in Tumor-Associated Macrophages. Adv. Mater. 2019, 31, 1904364.
- Perisé-Barrios, A.J.; Gómez, R.; Corbí, A.L.; De La Mata, J.; Domínguez-Soto, A.; Muñoz-Fernandez, M.A. Use of carbosilane dendrimer to switch macrophage polarization for the acquisition of antitumor functions. Nanoscale 2015, 7, 3857–3866.
- Liu, L.; Yi, H.; He, H.; Pan, H.; Cai, L.; Ma, Y. Tumor associated macrophage-targeted microRNA delivery with dual-responsive polypeptide nanovectors for anti-cancer therapy. Biomaterials 2017, 134, 166–179.
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132.
- Conde, J.; Bao, C.; Tan, Y.; Cui, D.; Edelman, E.R.; Azevedo, H.S.; Byrne, H.J.; Artzi, N.; Tian, F. Dual Targeted Immunotherapy via in Vivo Delivery of Biohybrid RNAi-Peptide Nanoparticles to Tumor-Associated Macrophages and Cancer Cells. Adv. Funct. Mater. 2015, 25, 4183–4194.
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front. Immunol. 2014, 5, 614.
- Parayath, N.N.; Parikh, A.; Amiji, M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018, 18, 3571–3579.
- Hu, A.; Chen, X.; Bi, Q.; Xiang, Y.; Jin, R.; Ai, H.; Nie, Y. A parallel and cascade control system: Magnetofection of miR125b for synergistic tumor-Association macrophage polarization regulation and tumor cell suppression in breast cancer treatment. Nanoscale 2020, 12, 22615–22627.
- Zhao, J.; Zhang, Z.; Xue, Y.; Wang, G.; Cheng, Y.; Pan, Y.; Zhao, S.; Hou, Y. Anti-tumor macrophages activated by ferumoxytol combined or surface-functionalized with the TLR3 agonist poly (I: C) promote melanoma regression. Theranostics 2018, 8, 6307–6321.
- Gong, T.; Song, X.; Yang, L.; Chen, T.; Zhao, T.; Zheng, T.; Sun, X.; Gong, T.; Zhang, Z. Spontaneously formed porous structure and M1 polarization effect of Fe3O4 nanoparticles for enhanced antitumor therapy. Int. J. Pharm. 2019, 559, 329–340.
- Chen, L.; Ma, X.; Dang, M.; Dong, H.; Hu, H.; Su, X.; Liu, W.; Wang, Q.; Mou, Y.; Teng, Z. Simultaneous T Cell Activation and Macrophage Polarization to Promote Potent Tumor Suppression by Iron Oxide-Embedded Large-Pore Mesoporous Organosilica Core–Shell Nanospheres. Adv. Healthc. Mater. 2019, 8, e1900039.
- Zhang, W.; Cao, S.; Liang, S.; Tan, C.H.; Luo, B.; Xu, X.; Saw, P.E. Differently Charged Super-Paramagnetic Iron Oxide Nanoparticles Preferentially Induced M1-Like Phenotype of Macrophages. Front. Bioeng. Biotechnol. 2020, 8, 537.
- Dorrington, M.G.; Fraser, I.D.C. NF-κB signaling in macrophages: Dynamics, crosstalk, and signal integration. Front. Immunol. 2019, 10, 705.
- Li, K.; Lu, L.; Xue, C.; Liu, J.; He, Y.; Zhou, J.; Xia, Z.; Dai, L.; Luo, Z.; Mao, Y.; et al. Polarization of tumor-associated macrophage phenotype: Via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale 2020, 12, 130–144.
- Liu, L.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. A Biomimetic Polymer Magnetic Nanocarrier Polarizing Tumor-Associated Macrophages for Potentiating Immunotherapy. Small 2020, 16, 2003543.
- Wei, B.; Pan, J.; Yuan, R.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Polarization of Tumor-Associated Macrophages by Nanoparticle-Loaded Escherichia coli Combined with Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2021, 21, 4231–4240.
- Shan, H.; Dou, W.; Zhang, Y.; Qi, M. Targeted ferritin nanoparticle encapsulating CpG oligodeoxynucleotides induces tumor-associated macrophage M2 phenotype polarization into M1 phenotype and inhibits tumor growth. Nanoscale 2020, 12, 22268–22280.
- Good, L.; Benner, B.; Carson, W.E. Bruton’s tyrosine kinase: An emerging targeted therapy in myeloid cells within the tumor microenvironment. Cancer Immunol. Immunother. 2021, 70, 2439–2451.
- Qiu, Q.; Li, C.; Song, Y.; Shi, T.; Luo, X.; Zhang, H.; Hu, L.; Yan, X.; Zheng, H.; Liu, M.; et al. Targeted delivery of ibrutinib to tumor-associated macrophages by sialic acid-stearic acid conjugate modified nanocomplexes for cancer immunotherapy. Acta Biomater. 2019, 92, 184–195.
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071.
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603.
- Chen, J.; Li, M.; Yang, C.; Yin, X.; Duan, K.; Wang, J.; Feng, B. Macrophage phenotype switch by sequential action of immunomodulatory cytokines from hydrogel layers on titania nanotubes. Colloids Surf. B Biointerfaces 2018, 163, 336–345.
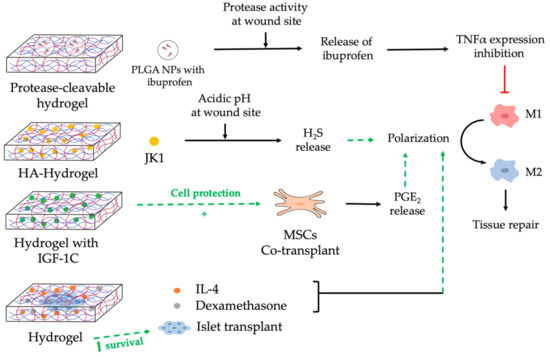
- Nguyen, D.T.; Soeranaya, B.H.T.; Truong, T.H.A.; Dang, T.T. Modular design of a hybrid hydrogel for protease-triggered enhancement of drug delivery to regulate TNF-α production by pro-inflammatory macrophages. Acta Biomater. 2020, 117, 167–179.
- Kang, J.; Neill, D.L.; Xian, M. Phosphonothioate-based hydrogen sulfide releasing reagents: Chemistry and biological applications. Front. Pharmacol. 2017, 8, 2–11.
- Miao, L.; Shen, X.; Whiteman, M.; Xin, H.; Shen, Y.; Xin, X.; Moore, P.K.; Zhu, Y.Z. Hydrogen Sulfide Mitigates Myocardial Infarction via Promotion of Mitochondrial Biogenesis-Dependent M2 Polarization of Macrophages. Antioxid. Redox Signal. 2016, 25, 268–281.
- Wu, J.; Chen, A.; Zhou, Y.; Zheng, S.; Yang, Y.; An, Y.; Xu, K.; He, H.; Kang, J.; Luckanagul, J.A.; et al. Novel H2S-Releasing hydrogel for wound repair via in situ polarization of M2 macrophages. Biomaterials 2019, 222, 119398.
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Jyothi Prasanna, S. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE 2 -dependent mechanism. Sci. Rep. 2016, 6, 152.
- Cao, X.; Duan, L.; Hou, H.; Liu, Y.; Chen, S.; Zhang, S.; Liu, Y.; Wang, C.; Qi, X.; Liu, N.; et al. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE2-mediated M2 macrophage polarization. Theranostics 2020, 10, 7697–7709.
- Zhao, X.; Cui, K.; Li, Z. The role of biomaterials in stem cell-based regenerative medicine. Future Med. Chem. 2019, 11, 1779–1792.
- Kumar, M.; Gupta, P.; Bhattacharjee, S.; Nandi, S.K.; Mandal, B.B. Immunomodulatory injectable silk hydrogels maintaining functional islets and promoting anti-inflammatory M2 macrophage polarization. Biomaterials 2018, 187, 1–17.




