
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | María Jesús Serrano Andrés | + 1402 word(s) | 1402 | 2021-08-18 10:35:18 | | | |
| 2 | Enzi Gong | Meta information modification | 1402 | 2021-08-23 11:01:57 | | |
Video Upload Options
Antimicrobial detection tests are conventional screening tools used in slaughterhouses to prevent the entry of antimicrobial residues into the food chain. The occasional appearance of antibiotic or bacteriostatic residues is a problem of major worldwide concern, as such residues can lead not only to toxicity for humans, but also to the emergence of antimicrobial resistance (AMR). In particular, antibacterial residues that contaminate meat can cause allergic reactions, can lead to dysbiosis of the gastrointestinal flora and can enhance dissemination of AMR, not only in the environment but also inside the gut, leading to antibacterially resistant communities in our intestinal flora.
1. Overview
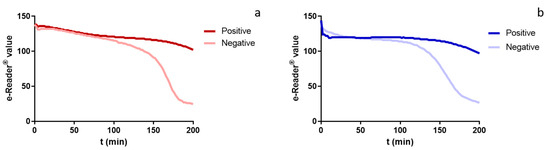
Even though antibiotics are necessary in livestock production, they can be harmful not only due to their toxicity, but also in view of their contribution to the emergence of antimicrobial resistance. Screening tests based on microbial growth inhibition appeared to be useful tools to prevent their entry into the food chain. They have nevertheless been traditionally carried out post mortem, leading to great economical loss and harm to the environment in case a positive sample is found. Hence, the objective was to evaluate the use of a screening test as an ante mortem alternative for the detection of antibiotic residues in meat: thus, Explorer®-Blood test was optimized and validated. After adapting the procedure for matrix preparation, the assay parameters were assessed from 344 antibiotic-free blood serum samples. Limits of Detection (LoDs) were defined by spiking blood serum with several of the most common antimicrobials used in veterinary practice. LoDs were similar to those obtained for meat and were at or below the maximum residue limits set by EU legislation for muscle. Analyses of in vivo injected samples, previously characterized by LC-MS/MS, demonstrated the method’s accuracy and proved that Explorer®-Blood can be considered a suitable alternative to conventional post mortem screening methods.
2. Antimicrobial Detection Tests
3. Conclusions
References
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514.
- Cheng, G.; Ning, J.; Ahmed, S.; Huang, J.; Ullah, R.; An, B.; Hao, H.; Dai, M.; Huang, L.; Wang, X.; et al. Selection and dissemination of antimicrobial resistance in Agri-food production. Antimicrob. Resist. Infect. Control. 2019, 8, 1–13.
- Lee, M.H.; Lee, H.J.; Ryu, P.D. Public health risks: Chemical and antibiotic residues—Review. Asian-Australas. J. Anim. Sci. 2001, 14, 402–413.
- Zdziarski, P.; Simon, K.; Majda, J.A.C.E.K. Overuse of high stability antibiotics and its consequences in public and environmental health. Acta Microbiol. Pol. 2003, 52, 5–13.
- Reig, M.; Toldrá, F. Veterinary drug residues in meat: Concerns and rapid methods for detection. Meat Sci. 2008, 78, 60–67.
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Ghering, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food. Chem. Toxicol. 2016, 88, 112–122.
- Nguyen, V.; Nguyen, V.; Li, C.; Zhou, G. The degradation of oxytetracycline during thermal treatments of chicken and pig meat and the toxic effects of degradation products of oxytetracycline on rats. J. Food Sci. Technol. 2015, 52, 2842–2850.
- Elzagallaai, A.A.; Sultan, E.A.; Bend, J.R.; Abuzgaia, A.M.; Loubani, E.; Rieder, M.J. Role of oxidative stress in hypersensitivity reactions to sulfonamides. J. Clin. Pharmacol. 2020, 60, 409–421.
- Commission Regulation (EU). No. 726/2004 of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. Off. J. Eur. Union 2004, L. 136, 1–33.
- Commission Regulation (EU). No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, L. 15, 1–72.
- Directive 2001/82/EC of the European parliament and of the council of 6 November 2001 on the Community code relating to veterinary medicinal products. Off. J. Eur. Community 2001, L. 311, 1–66.
- Council Directive (EEC). No. 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products. Off. J. Eur. Community 1996, L. 125, 1–32.
- Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and interpretation of results. Off. J. Eur. Community 2002, L. 221, 1–36.
- Pikkemaat, M.G. Microbial screening methods for detection of antibiotic residues in slaughter animals. Anal. Bioanal. Chem. 2009, 395, 893–905.
- Mata, L.; Sanz, D.; Razquin, P. Validation of the Explorer® 2.0 test coupled to e-Reader® for the screening of antimicrobials in muscle from different animal species. Food Addit. Contam. Part A 2014, 31, 1496–1505.
- Gaudin, V.; Rault, A.; Hedou, C.; Soumet, C.; Verdon, E. Strategies for the screening of antibiotic residues in eggs: Comparison of the validation of the classical microbiological method with an immunobiosensor method. Food Addit. Contam. Part A 2017, 34, 1510–1527.
- Djekic, I.; Radović, Č.; Lukić, M.; Stanišić, N.; Lilić, S. Environmental life-cycle assessment in production of pork products. Meso 2015, 17, 345–351.
- Jones, S.A.; Salter, R.S.; Goldsmith, T.; Quintana, J.; Rapnicki, P.; Shuck, K.; Wells, J.E.; Schneider, M.J.; Griffin, D. Development and model testing of antemortem screening methodology to predict required drug withholds in heifers. J. Food Prot. 2014, 77, 292–298.
- Wu, Q.; Zhu, Q.; Shabbir, M.A.B.; Sattar, A.; Peng, D.; Tao, Y.; Chen, D.; Yuan, Z.; Wang, Y. The search for a microbiological inhibition method for the rapid, broad-spectrum and high-throughput screening of six kinds of antibiotic residues in swine urine. Food Chem. 2021, 339, 127580.
- Hernández, E.; Rey, R.; Puig, M.; Garcia, M.A.; Solans, C.; Bregante, M.A. Pharmacokinetics and residues of a new oral amoxicillin formulation in piglets: A preliminary study. Vet. J. 2005, 170, 237–242.
- Reyes-Herrera, I.; Schneider, M.J.; Cole, K.; Farnell, M.B.; Blore, P.J.; Donoghue, D.J. Concentrations of antibiotic residues vary between different edible muscle tissues in poultry. Research note. J. Food Prot. 2005, 68, 2217–2219.
- Castellari, M.; Gratacos-Cubarsi, M.; Garcia-Regueiro, J.A. Detection of tetracycline and oxytetracycline residues in pig and calf hair by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8096–8100.
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Antibiotic use in heavy pigs: Comparison between urine and muscle samples from food chain animals analysed by HPLC-MS/MS. Food Chem. 2017, 235, 111–118.
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Suitability of feathers as control matrix for antimicrobial treatments detection compared to muscle and liver of broilers. Food Control 2018, 91, 268–275.
- Serrano, M.J.; Mitjana, O.; Bonastre, C.; Laborda, A.; Falceto, M.V.; García-Gonzalo, D.; Abilleira, E.; Elorduy, J.; Bousquet-Melou, A.; Mata, L.; et al. Is Blood a Good Indicator for Detecting Antimicrobials in Meat? Evidence for the Development of In Vivo Surveillance Methods. Antibiotics 2020, 9, 175.




