
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Piccioni | + 1722 word(s) | 1722 | 2021-08-09 06:29:20 | | | |
| 2 | Vivi Li | Meta information modification | 1722 | 2021-08-20 09:34:21 | | |
Video Upload Options
The microbiota is the set of commensal microorganisms, residing in the organism, helping proper functioning of organs and systems. The role that the microbiota plays in maintaining the health of vertebrates is widely accepted, particularly in the gastrointestinal system, where it is fundamental for immunity, development, and conversion of nutrients. Dysbiosis is an alteration of the microbiota which refers to a disturbed balance, which can cause a number of pathologies. Probiotics have proven to be effective in modulating the microbiota of the gastrointestinal system and, therefore, in promoting the health of the individual. In particular, Lactobacilli are a group of Gram-positive bacteria, which are able to produce lactic acid through glucose metabolism. They are present in different microenvironments, ranging from the vagina, to the mouth, to different tracts of the small intestine.
1. Introduction
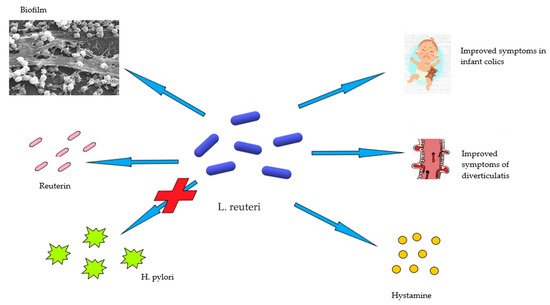
2. Limosilactobacillus Reuteri in Human Health and Disease
3. Acute Diverticulitis
References
- Giraffa, G.; Chanishvili, N.; Widyastuti, Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010, 161, 480–487.
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757.
- Macklaim, J.M.; Clemente, J.C.; Knight, R.; Gloor, G.B.; Reid, G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 2015, 26, 27799.
- Romani Vestman, N.; Chen, T.; Lif Holgerson, P.; Öhman, C.; Johansson, I. Oral Microbiota Shift after 12-Week Supplementation with Lactobacillus reuteri DSM 17938 and PTA 5289; A Randomized Control Trial. PLoS ONE 2015, 10, e0125812.
- Talarico, T.L.; Dobrogosz, W.J. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1989, 33, 674–679.
- Gänzle, M.G.; Vogel, R.F. Studies on the mode of action of reutericyclin. Appl. Environ. Microbiol. 2003, 69, 1305–1307.
- Yang, F.; Wang, A.; Zeng, X.; Hou, C.; Liu, H.; Qiao, S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 32.
- Thomas, C.M.; Hong, T.; van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE 2012, 7, e31951.
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242.
- Salas-Jara, M.J.; Ilabaca, A.; Vega, M.; García, A. Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms 2016, 4, 35.
- Kšonžeková, P.; Bystrický, P.; Vlčková, S.; Pätoprstý, V.; Pulzová, L.; Mudroňová, D.; Kubašková, T.; Csank, T.; Tkáčiková, Ľ. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19.
- Cervantes-Barragan, L.; Chai, J.N. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8αα(+) T cells. Science 2017, 357, 806–810.
- Franceschi, F.; Cazzato, A.; Nista, E.C.; Scarpellini, E.; Roccarina, D.; Gigante, G.; Gasbarrini, G.; Gasbarrini, A. Role of probiotics in patients with Helicobacter pylori infection. Helicobacter 2007, 12 (Suppl. 2), 59–63.
- Francavilla, R.; Polimeno, L.; Demichina, A.; Maurogiovanni, G.; Principi, B.; Scaccianoce, G.; Ierardi, E.; Russo, F.; Riezzo, G.; Di Leo, A.; et al. Lactobacillus reuteri strain combination in Helicobacter pylori infection: A randomized, double-blind, placebo-controlled study. J. Clin. Gastroenterol. 2014, 48, 407–413.
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598.
- Mu, Q.; Zhang, H.; Liao, X.; Lin, K.; Liu, H.; Edwards, M.R.; Ahmed, S.A.; Yuan, R.; Li, L.; Cecere, T.E.; et al. Control of lupus nephritis by changes of gut microbiota. Microbiome 2017, 5, 73.
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e7.
- Mi, G.L.; Zhao, L.; Qiao, D.D.; Kang, W.Q.; Tang, M.Q.; Xu, J.K. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: A prospective single blind randomized trial. Antonie Van Leeuwenhoek 2015, 107, 1547–1553.
- Violi, A.; Cambiè, G.; Miraglia, C.; Barchi, A.; Nouvenne, A.; Capasso, M.; Leandro, G.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F. Epidemiology and risk factors for diverticular disease. Acta Bio-Med. Atenei Parm. 2018, 89 (Suppl. 9), 107–112.
- Munie, S.T.; Nalamati, S.P.M. Epidemiology and Pathophysiology of Diverticular Disease. Clin. Colon Rectal Surg. 2018, 31, 209–213.
- Schieffer, K.M.; Kline, B.P.; Yochum, G.S.; Koltun, W.A. Pathophysiology of diverticular disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 683–692.
- McSweeney, W.; Srinath, H. Diverticular disease practice points. Aust. Fam. Physician 2017, 46, 829–832.
- Bernades, P. Natural history of diverticular disease of the colon. Ann. Gastroenterol. D’Hepatol. 1986, 22, 209–211.
- Thompson, A.E. Diverticulosis and Diverticulitis. JAMA 2016, 316, 1124.
- Feuerstein, J.D.; Falchuk, K.R. Diverticulosis and Diverticulitis. Mayo Clin. Proc. 2016, 91, 1094–1104.
- Tursi, A.; Papa, A.; Danese, S. Review article: The pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment. Pharmacol. Ther. 2015, 42, 664–684.
- Strate, L.L.; Morris, A.M. Epidemiology, Pathophysiology, and Treatment of Diverticulitis. Gastroenterology 2019, 156, 1282–1298.e1.
- Swanson, S.M.; Strate, L.L. Acute Colonic Diverticulitis. Ann. Intern. Med. 2018, 168, Itc65–itc80.
- Germer, C.T. Diverticular disease of the colon. Der Chir. Z. Fur Alle Geb. Der Oper. Medizen 2014, 85, 280.
- Bharucha, A.E.; Parthasarathy, G.; Ditah, I.; Fletcher, J.G.; Ewelukwa, O.; Pendlimari, R.; Yawn, B.P.; Melton, L.J.; Schleck, C.; Zinsmeister, A.R. Temporal Trends in the Incidence and Natural History of Diverticulitis: A Population-Based Study. Am. J. Gastroenterol. 2015, 110, 1589–1596.
- Gargallo Puyuelo, C.J.; Sopeña, F.; Lanas Arbeloa, A. Colonic diverticular disease. Treatment and prevention. Gastroenterol. Y Hepatol. 2015, 38, 590–599.
- Stollman, N.; Magowan, S.; Shanahan, F.; Quigley, E.M. A randomized controlled study of mesalamine after acute diverticulitis: Results of the DIVA trial. J. Clin. Gastroenterol. 2013, 47, 621–629.




