Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alice Vilela | + 3283 word(s) | 3283 | 2021-08-10 05:00:33 | | | |
| 2 | Peter Tang | Meta information modification | 3283 | 2021-08-11 03:44:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vilela, A. Grape Infusions. Encyclopedia. Available online: https://encyclopedia.pub/entry/13018 (accessed on 08 February 2026).
Vilela A. Grape Infusions. Encyclopedia. Available at: https://encyclopedia.pub/entry/13018. Accessed February 08, 2026.
Vilela, Alice. "Grape Infusions" Encyclopedia, https://encyclopedia.pub/entry/13018 (accessed February 08, 2026).
Vilela, A. (2021, August 10). Grape Infusions. In Encyclopedia. https://encyclopedia.pub/entry/13018
Vilela, Alice. "Grape Infusions." Encyclopedia. Web. 10 August, 2021.
Copy Citation
Grape-infusion preparation is no more than a sustainable or green way of extracting polyphenols and other nutraceutical compounds from grapes and grape leaves. Grapes and grape/wine by-products are a rich source of health-promoting compounds, presenting great potential for the development of new beverages.
grape berry
grape leaves
polyphenols
nutraceutical compounds extraction
anthocyanins
trans-resveratrol
quercetin and proanthocyanidins
sustainable chemistry
1. Introduction
Wineries produce a large amount of waste during the grape-harvesting and winemaking periods. It is estimated that 1 billion kg/y of grape stalks are produced worldwide [1] and the total amount of solid waste produced, after winemaking, may well account for over 30% (w/w) of the grapes used [2].
For some time now, there has been a greater demand for sustainable food production. Low use of inputs, zero waste, aggregation of social values, and minimization of environmental impact are essential premises for food production in the world today. To implement sustainable practices in the wine industry, and in all its by-products, aiming to achieve a “Greener Enology/Viticulture”, it is necessary to fully understand the constitution of the plants and Enological/Viticultural processes to better profit from their use, in an environmentally friendly way.
Vitis vinifera L. is a species that has always accompanied man over time [3][4]. It is considered one of the most important cultures in the world (77.8 million tons in the world production of grapes in 2018 from 7.4 million hectares [5]), both in terms of economic valuation and occupied area [6]. Wine and spirits are the main products of the vine industry. However, they are not the only products. Thus, fresh grapes, raisins, juices, jellies, molasses, jam, and dishes cooked with vine leaves, are also foodstuffs with nutraceutical characteristics produced from the approximately 5000 cultivars of Vitis vinifera used in the grape industry [4][7][8]. As an example of benefits related to human health, several studies cited by Cosme et al. [9] in grapes and their derivatives, where anti-inflammatory, anticancer, antimicrobial, antiviral, cardioprotective, neuroprotective, and hepatoprotective activities are attributed.
Vitis vinifera is a climbing species (Figure 1), where different organs are recognized, such as roots, trunk, cordon, shoots, leaves, tendrils, and berries [10][11].
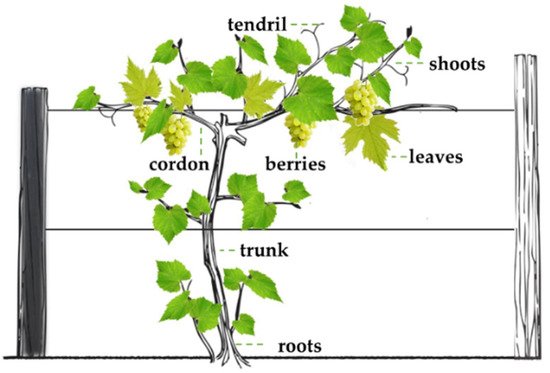
Figure 1. Grapevine structures.
All parts of this plant have ingredients that are essential for making an infinite variety of value-added products. However, we are going to focus our work on grape berries and leaves once they are the most profitable organs of this species and are described below.
1.1. Grape Berry
The vine bear fruit in a cluster (Figure 2A). The fruit is botanically called berry as it reserves the seeds protected by a pericarp inside [11].
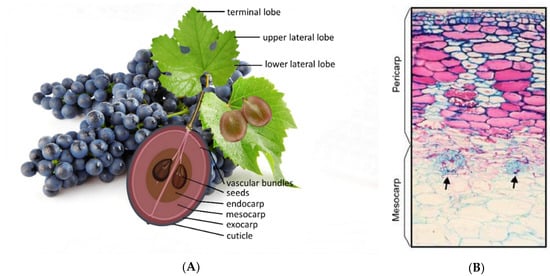
Figure 2. (A) Vine cluster with leaves and a schematic structure of a grape berry. (B) Anatomical section of pericarp and mesocarp of the grape berry. Arrows indicate the peripheral carpellary bundles. Adapted from Zhang et al. [12].
Depending on the cultivars, the size and shape of the berries vary greatly. The berries may have a rounded, ovoid, oblate, or elliptical shape [10]. The changes in color, size, aroma, flavor, and texture, observed throughout the development of the berry, are due to changes that occur in the contents of the cell vacuoles [13]. With an integrated structure, three different layers of tissue can be observed in the berries:
-
Exocarp—includes the cuticle (a waxy layer), epidermis, and hypodermis, consisting of 6 to 8 layers of cells smaller in size than the mesocarp cells [8]. However, between 5 to 18% of the fresh weight of the berry is attributed to the skin [14][15]. The epidermis is formed by tangentially elongated cells, one or two layers. The hypodermis can represent four to five layers of cells, in which the outermost layers of the cells have a rectangular shape, as opposed to the innermost layers in which the cells are polygonal [8][16].
-
Mesocarp (Figure 2B)—rich in anthocyanins in red cultivars, occupies between 85–87% of the grape volume. Is made up of rounder and polygonal cells, with thin cell walls, very vacuolized [9][17]. It is exactly in these organelles, the vacuoles, where it is possible during the ripening of the grape to find sugars, organic acids, water, and ions [13].
1.2. Grape Leaves
Although the grapevine is a particularly important crop in the world economy, and the use of grape leaves in gastronomy is already very ingrained in some cultures, namely in Europe, North Africa, and the Middle East [4][8][20], it would be important to foster new ways of valuing this nutritionally rich by-product [21][22][23]. Thus, knowledge of the ultrastructure of the leaves of the different cultivars is important to assess the viability of their use in human food. According to Vilela and Pinto [24], some varieties of Vitis vinifera have crystals of calcium oxalate in the leaves, making its use unfeasible for human consumption, due to the potential risk of causing kidney stones [25].
Typically, the vine leaf has a hand-like shape, with three lobes (Figure 3A) [26][27][28], although its shape may vary with the cultivar. With a heterogeneous mesophyll, it is easily recognized that the upper surface of the leaf is structurally different from the lower surface. Thus, associated with the upper surface the palisade chlorophyll parenchyma is identified, where the cells are rod-shaped, close together and with few spaces between them. The lower surface also allocates chlorophyll parenchyma, but with round cells with an irregular contour and many intercellular air spaces, named spongy parenchyma (Figure 3B,C). This difference in the structure of the chlorophyll parenchyma associated with the two pages of the leaf implies an uneven distribution of chlorophyll on both sides of the leaf. Thus, the leaf shows different coloring on the two pages, darker green on the upper surface and light green on the lower surface [29]. The entire leaf is traversed by a vascular system organized in bundles composed of xylem and phloem (Figure 3B,C), with greater representation in the main vein. Associated with these, it is still possible to identify support tissue, mostly fibers [8] The entire leaf is covered externally by a protective tissue, the epidermis, where stomata are mostly distributed on the lower surface.
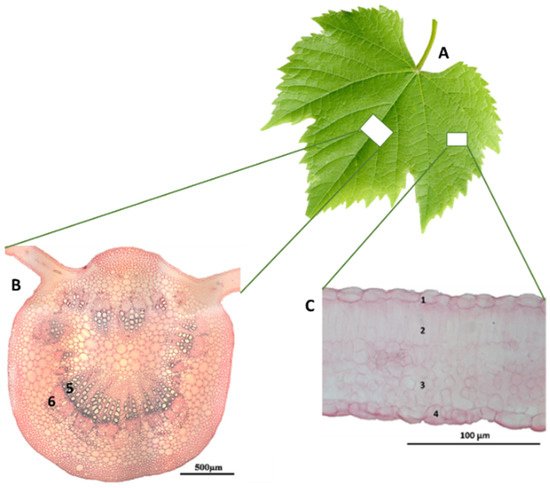
Figure 3. Vitis vinifera leaf (A), a cross-section of the main vein (B) and mesophyll (C). 1, Upper epidermal cells; 2, palisade parenchyma; 3, spongy parenchyma; 4, lower epidermal cells; 5, xylem; 6, phloem. Adapted from Cosme et al. [8].
2. Nutraceutical Compounds of Grape Berries and Grape-Leaves
The economic importance of grape and wine production in many countries is widely recognized. In the 21st century, world wine production has remained relatively stable. In 2016, it reached 267 million hectoliters, with geography very dispersed throughout the world, with Europe holding a dominant position with 60% of production, Figure 4 [30].
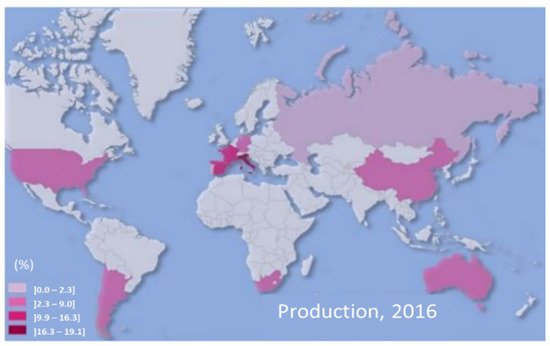
Figure 4. World geography of wine and grape production in 2016. Adapted from Hogg and Rebelo [30].
The relevance of grape production and its by-products is not limited to economic factors, but also its nutritional and functional properties. This material is considered a rich source of bioactive compounds, thus being of interest to the cosmetics, pharmaceutical, and food industries.
The phenolic compound’s potential protective role has been widely studied. Its antioxidant properties are attributed to its ability to remove free radicals and inhibit the formation of reactive species during metabolic processes, to prevent damage to biomolecules such as lipids, proteins, and nucleic acids, contributing to the prevention of cell damage [31][32]. Even more, it may involve block cell propagation and apoptosis as well [33].
Polyphenols are a major class of bioactive phytochemicals [33]. Cassidy and co-workers [34] estimated the existence of more than 8000 known phenolic compounds, which include several subclasses such as phenolic acids, flavonoids, stilbenes, lignans, and tannins.
Structurally based on a 15-carbon skeleton arranged in three rings (C6-C3-C6) (see Figure 5) [31], flavonoids comprise a large group of phenolic compounds, including flavan-3-ols, flavonols, flavones, isoflavones, flavanones, and anthocyanins [35].
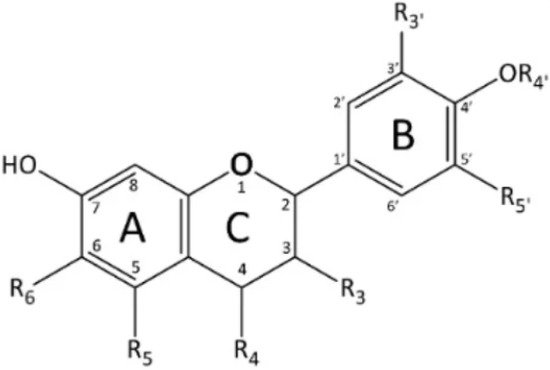
Figure 5. The basic structure of flavonoids. A and B—benzene rings; C—closed pyran ring.
Most flavonoids are transformed in the small intestine before being absorbed. Although most ingested flavonoids are absorbed in the small intestine, there is also significant absorption in the large intestine [36][37]. Readers should note the example of green tea in which, according to Selma et al. [38], a part of its flavonoids is metabolized in the small intestine before being absorbed, and another part is metabolized by the large intestine microbiota forming phenolic acids. Crozier et al. [36] also mention that the colon bacteria influence the absorption of several flavonoids, which may determine their bioavailability in the systemic circulation.
Among the various foods rich in flavonoids are tea, grapes, and red wine [32]. The grape seed extract was recently shown to improve the disturbance and metabolic function of intestinal flora in rats [39]. Several studies showed that ingestion of flavan-3-ol from various dietary sources has positive effects on cardiovascular disease, reducing the risk of diabetes, cholesterol levels, blood pressure [40][41]. What is more, Arts et al. [42] found an inverse relationship between coronary heart disease and consumption of flavanols, flavonols, and flavones. Peter et al. [43] report an 11% reduction in the risk of cardiovascular disease demonstrated by the ingestion of three cups of tea a day. Age-related vascular problems can also be avoided by consuming flavanols and flavonols [44]. Anthocyanins, as with other polyphenols, also have anti-inflammatory properties and positive vascular effects [34]. Indeed, ingestion of flavonoids and anthocyanins has led to reduced mortality related to cardiovascular disease [45].
Over the past two years, many meta-analyses have shown a positive relationship between cardiovascular disease and COVID-19 disease severity [46][47][48][49][50]. On the other hand, resveratrol is known for its antiviral effects against several respiratory tract viruses [51] including MERS-CoV [52]. So, several studies have already anticipated the potential implementation of resveratrol in the treatment of COVID-19. Yang et al. [53] demonstrated that resveratrol inhibits SARS-CoV-2 replication in cultured Vero cells and, as a result, its potential utility as a new therapy has been proposed. Wahedi et al. [54] recognized that resveratrol is a promising candidate for drug development against COVID-19. Furthermore, with its proven antithrombotic effects, resveratrol has been proposed as an adjunct treatment to delay and improve vascular thrombosis in the course of COVID-19 [55]; it has a beneficial effect on the modulation of inflammation without compromising the adaptive immune response [56]. Given its antioxidant effect, it may also contribute to the prevention of viral protein binding in host cells [57]. The association of resveratrol with essential minerals is beneficial in reducing the mortality of patients with COVID-19 [58]. Resveratrol is also recognized as a cardioprotective supplement for mitigating cardiotoxicity associated with chloroquine/hydroxychloroquine treatment in SARS-CoV-2 patients, enhancing its antiviral potency [59].
In different concentrations, grape berry tissues are still rich in other types of compounds [60]:
-
Sugars such as glucose, fructose, and sucrose.
-
Organic acids such as malic, tartaric, and citric acid.
-
Aroma precursors, which could be volatile and non-volatile.
In addition to its wide use in the gastronomy of some cultures, the leaves of the vine have also been used for medical purposes, such as to stop bleeding, inflammation, pain, and diarrhea [61]. Leaves, usually discarded by grape farmers, are a rich source of vitamins, minerals, crude fiber, and phenolic compounds [4][62].
Flavonols [63], caffeic acid [64], hydroxycinnamic tartaric esters, catechin, quercetin, rutin, and kaempferol [4] are compounds that can be found in leaves. However, according to Lacerda et al. [65], the acid gallic acid is the dominant phenolic acid in the vine leaf.
Resveratrol and some of its derivatives are present in various extracts of grape leaves, regardless of variety. Trans- and cis-resveratrol and astringin (a stilbenoid), are the stilbenes that can be mentioned because they are present in some red grape varieties such as Syrah, Merlot, Vranac, and white varieties such as Marastina and Posip [66]. The phase of the plant’s vegetative cycle is also a determining factor in the concentration of phenolic compounds, increasing with vegetative development: May (2911 mgL−1 gallic acid equivalents), September (3339 mgL−1 gallic acid equivalents) [66].
Additionally, Monagas et al. [67] when studying the composition of the vine leaf, identified anthocyanins (delphinidin, cyanidin, petunidin, peonidin, and malvidin-3-glucosides and -3-(6-p-coumaroyl)-glucosides, and cyanidin and peonidin-3-(6-acetyl)-glucosides), and non-anthocyanin compounds (trans-caftaric acid, and the -3-O-galactoside, -glucuronide and -glucoside derivatives of quercetin and kaempferol, and quercetin aglycone).
3. Ways of Extracting Nutraceutical Compounds from Grapes and Grapes by-Products
Grape marc, fermented or semi-fermented, is constituted by high contents of biodegradable compounds and solid by-products, consisting mainly of grape stalks, seeds, skins, and wine lees. Grape marc is either sent to distilleries for obtaining ethanol or grape-spirit, also called “Aguardente Velha” or Cognac when submitted to double distillation, rectification, and wood-aging [68], or is discarded as natural waste. Recently, the productive use of such by-products can offer economic advantages once they constitute an abundant feedstock for biodiesel production in wine-producing countries [69], or they can be used—due to a high level of polyphenols that possess antioxidant and radical scavenging activities [70][71]—to obtain nutraceutical compounds, such as polyphenols, used in food supplementation [72], in new nutraceutical beverages formulations [73], in pharmaceutical formulations [74] and even in cosmetic products [75].
To avoid spoilage and time-degradation of grape marc, drying is used as a preservation method, inhibiting the growth of microorganisms, and delaying chemical reactions [76][77]. Natural solar drying, or, when the sun is not available, hot-air drying is the most used method to produce dried enological and agricultural products due to the smaller operating cost. However, phenolic, and other nutraceutical compounds, that can be extracted from these biological wastes are heat-sensitive substances [78], therefore temperatures lower than 60–70 °C are considered suitable for the pre-treatment of grape marc and other agricultural by-products to retain its bioactive compounds [24][79].
In the case of grape marc, when the focus compounds are soluble or weakly bound, such as polyphenols, the most common technique of extraction is solid-liquid extraction, mainly based on organic solvents (SLE) [80]. The most used procedures, in the laboratory, are mechanical agitation and Soxhlet extraction (SOX) (Figure 6). In the 1st step (Figure 6A) the solid matrix is placed in SOX thimble and the solvent is heated under reflux. Then, condensation and extraction with “fresh” solvent is carried out. Solutes are transferred from the extraction chamber into the reservoir (Figure 6B). Continuous repetition of the extraction occurs (Figure 6C). Finally, exhaustive extraction is complete (Figure 6D) [81]. The total extraction time ranges between 12–24 h, making this a time-consuming technique. Additionally, the most common extractors use large amounts of purified solvent (several hundred milliliters) causing potential environmental pollution [81][82].
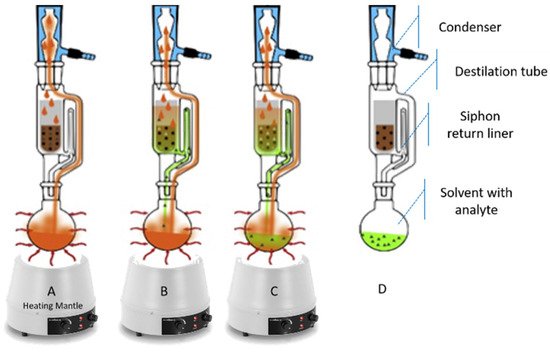
Figure 6. Schematic illustration of the workflow of SOX. Adapted from Weggler et al. [81]. (A) The solid matrix is placed in SOX thimble and the solvent is heated under reflux; (B) Solutes are transferred from the extraction chamber into the reservoir; (C) Continuous repetition of the extraction occurs; (D) Exhaustive extraction is complete.
Solid-liquid extraction at a semi-industrial scale can be performed using ethanol/water solutions. Escobar-Avello et al. [83] worked with grape canes (vine shoots), a viticultural by-product containing high levels of phenolic compounds. They used grape canes of Vitis vinifera cv. Pinot noir, and a 750 L pilot-plant reactor under the following conditions: T = 80 °C, time 100 min, solid/liquid ratio 1:10. The extraction solvent was constituted by ethanol: water solution (80:20, v/v).
Other solid-liquid extraction solvents, including green solvents and methods, will be referred to further in this manuscript.
Due to the disadvantages presented (loss of compounds due to hydrolysis, oxidation of the target compounds during extraction, long extraction time, and environmental pollution [84] this technique, and other similar ones, tend to be replaced by novel extraction methodologies including mechanical extraction by ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) [1], and greener technologies such as supercritical fluid extraction (SFE) and pressurized liquid extraction (PLE) [85]. The latter can achieve high yields of bioactive compounds with the use of food-grade and non-toxic solvents [86][87]. Degrading enzyme mixtures of the grapes cell wall polysaccharide can also be used, leading to the recovery of compounds with bioactive properties [88][89].
4. Grape Infusions as a Way of Extracting Nutraceutical and Antimicrobial Compounds
If an herbal formulation should generate far less or even zero wastes, otherwise the very essence of sustainability is canceled, an infusion made of grape berries or leaves should follow the same principle. In this regard, a solid-liquid extraction of bioactive substances from medicinal plants should embrace methodologies characterized by using water as a solvent. That is the case with grape infusions. Dried non-fermented/semi-fermented and even fermented grapes, skins, and seeds can be used in the preparation of infusions or tisanes [24].
To prepare the dried vegetal by-product for infusion preparation, the most utilized drying methods are hot-air/oven drying, low temperature-air drying, and freeze-drying [24][90][91]. Grape by-products can simply be placed into trays and dried in ovens with controlled temperature and air circulation. Then, the dry material (non-fermented/semi-fermented or fermented grapes, skins, and seeds) is crushed using a mincer and the minced material can then be used to prepare infusions by adding water at a temperature of about 95–100 °C (Figure 7A–D).

Figure 7. Dehydrated berries at 60 °C (A) and crushed (B); Dehydrated berries at 70 °C (C) and crushed (D); Lyophilized berries (E). The appearance of infusions prepared from dried berries at 60 °C (E) and 70 °C (F).
The drying temperature of the grapes influences the infusion’s chemical and sensory characteristics, including color [24] (Figure 7E,F). Yet, the optimal drying conditions for the storage of grape pomace, for further application, needs to be more investigated in order to optimize the extraction of phenolics. Gerardi et al. [92] performed the drying by oven at 50 °C which allowed the storage of grape skin from pomace, making it a fast and reproducible method.
Recently, Goulas et al. [93] studied the development of a functional infusion from the “Commandaria” grape pomace. They varied the brewing parameters such as the ratio of water to grape pomace powder, infusion time, and infusion temperature. Interestingly, brewing 200 mL of water per g of dried material for 12.2 min at 95 °C was the optimum method for preparing the infusions. The infusions also presented antimicrobial activity against Listeria monocytogenes serotypes S. enterica, S. aureus, and E. coli bacteria. Moreover, minimum inhibitory concentration (MIC) of 0.25–0.5 mg mL−1, concentrations such as a cup of infusion, was able to prevent the growth of some Listeria serotypes.
Bekhit Ael-D et al. [94] aiming at studying the antioxidant activities, sensory and anti-influenza activity of grape skin tea infusion found that low antioxidant recovery was obtained using hot water, however, the extracts maintained their antiviral activity. They concluded that grape skin extracts could be incorporated in different functional health-promoting beverages. Moreover, the use of ingredients such as green tea improved the antioxidant activity and the final infusion sensory profile.
Vine leaf infusion may also be an alternative for the valorization of vine leaves after the grape harvest. The leaves are usually left on the vine; in the autumn they fall and are used as organic material for natural fertilization. The possible use of vine leaves in herbal infusions depends on their aroma profile, as well as on their mouth-feel attributes, and, of course, their potential benefit for human health. According to HMPC [95] for the grape-leaf extract preparation (Extractum Vitis vinifera foliae aquosum siccum, 4–6:1), dried leaves of red varieties of Vitis vinifera L. (Vitis vinifera folium) which comply with the monograph described in Pharmacopée Française for “Vigne rouge” are used. Thus, the herbal substance consists of the dried leaves of the black to pulp-red grape which finally undergoes a specific production process resulting in defined flavonol content in the dry extract preparation. While the whole dry extract preparation as such is considered as an active agent, it is particularly characterized by its content of flavonol glycosides and glucuronides, i.e., quercetin-3-O-β-D-glucuronide, quercetin-3-O-β-glucoside, and kaempferol-3-glucoside. These flavonoids are considered to contribute predominantly to pharmacological effects. One water extract of red vine leaf contains a total of 4–7% of flavonol glycosides, quantified as quercetin-3-O-β-D-glucuronide. Several authors have studied and reported phenolic composition [96][97]; antioxidant activity [96][98], antimicrobial properties [99][100], and volatile composition [101] of Vitis vinifera L. leaves.
References
- Grillo, G.; Boffa, L.; Talarico, S.; Solarino, R.; Binello, A.; Cavaglià, G.; Bensaid, S.; Telysheva, G.; Cravotto, G. Batch and Flow Ultrasound-Assisted Extraction of Grape Stalks: Process Intensification Design up to a Multi-Kilo Scale. Antioxidants 2020, 9, 730.
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132.
- Vivier, M.A.; Pretorius, I.S. Genetic Improvement of Grapevine: Tailoring Grape Varieties for the Third Millennium—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 5–26.
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. In Vitro 2010, 24, 148–153.
- OIV, International Organisation of Vine and Wine Intergovernmental Organisation. Statistical Report on World Vitiviniculture. 2019. Available online: https://www.oiv.int/en/oiv-life/oiv-2019-report-on-the-world-vitivinicultural-situation (accessed on 4 June 2021).
- Torregrosa, L.; Vialet, S.; Adivèze, A.; Iocco-Corena, P.; Thomas, M.R. Grapevine (Vitis vinifera L.). Methods Mol. Biol. 2015, 1224, 177–194.
- Jackson, R.S. Wine Science Principles and Applications, 4th ed.; Academic Press: New York, NY, USA, 2014; ISBN 9780123814692.
- Cosme, F.; Pinto, T.; Vilela, A. Oenology in the Kitchen: The Sensory Experience Offered by Culinary Dishes Cooked with Alcoholic Drinks, Grapes, and Grape Leaves. Beverages 2017, 3, 42.
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22.
- Magalhães, N. Viticulture Treaty: The Vine, the Vineyard and the Terroir; Chaves Ferreira Publications: Lisboa, Portugal, 2008; ISBN 9728987153.
- Keller, M. The Science of Grapevines: Anatomy and Physiology, 3rd ed.; Academic Press: London, UK, 2020; ISBN 978-0-12-816365-8.
- Zhang, X.Y.; Wang, X.L.; Wang, X.F.; Xia, G.H.; Pan, Q.H.; Fan, R.F.; Wu, F.Q.; Yu, X.C.; Zhang, P. A Shift of Phloem Unloading from Symplasmic to Apoplasmic Pathway Is Involved in Developmental Onset of Ripening in Grape Berry. Plant Physiol. 2006, 142, 220–232.
- Kuang, L.; Chen, S.; Guo, Y.; Ma, H. Quantitative Proteome Analysis Reveals Changes in the Protein Landscape During Grape Berry Development with a Focus on Vacuolar Transport Proteins. Front. Plant Sci. 2019, 10, 641.
- Fontes, N.; Gerós, H.; Delrot, S. Grape Berry Vacuole: A Complex and Heterogeneous Membrane System Specialized in the Accumulation of Solutes. Am. J. Enol. Vitic. 2011, 62, 270–278.
- Wilson, B.; Strauss, C.R.; Williams, P.J. The distribution of free and glycosidically-bound monoterpenes among skin, juice, and pulp fractions of some white grape varieties. Am. J. Enol. Vitic. 1986, 37, 107–111.
- Schlosser, J.N.; Olsson, M.; Weis, K.; Reid, F.; Peng, S.; Lund, P.B. Expansão celular e expressão gênica na uva em desenvolvimento (Vitis vinifera L.). Protoplasma 2008, 232, 255–265.
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. The Microbiology of Wine and Vinifications. In Handbook of Enology, 1st ed.; Wiley: Chichester, UK, 2000; Volume 1.
- Hardie, W.J.; O’Brien, T.P.; Jaudzems, V.G. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust. J. Grape Wine Res. 1996, 2, 97–142.
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503.
- Güler, A.; Candemir, A. Total Phenolic and Flavonoid Contents, Phenolic Compositions, and Color Properties of Fresh Grape Leaves. Türk Tarım Doğa Bilim. Derg. 2014, 6, 778–782.
- Dogan, Y.; Nedelcheva, A.; Łuczaj, Ł.; Drăgulescu, C.; Stefkov, G.; Maglajlić, A.; Ferrier, J.; Papp, N.; Hajdari, A.; Mustafa, B.; et al. Of the importance of a leaf: The ethnobotany of sarma in Turkey and the Balkans. J. Ethnobiol. Ethnomed. 2015, 11, 26.
- El, S.N.; Kavas, A.; Karakaya, S. Nutrient Composition of Stuffed Vine Leaves: A Mediterranean Dietary. J. Food Qual. 1997, 20, 337–341.
- Romero, M.J.; Madrid, J.; Hernández, F.; Cerón, J.J. Digestibility and voluntary intake of vine leaves (Vitis vinifera L.) by sheep. Small Rumin. Res. 2000, 38, 191–195.
- Vilela, A.; Pinto, T. Grape Infusions: The Flavor of Grapes and Health-Promoting Compounds in Your Tea Cup. Beverages 2019, 5, 48.
- Singh, V.K.; Jaswal, B.S.; Sharma, J.; Rai, P.K. Analysis of stones formed in the human gall bladder and kidney using advanced spectroscopic techniques. Biophys. Rev. 2020, 12, 647–668.
- Boso, S.; Gago, P.; Alonso-Villaverde, V.; Santiago, J.J.; Mendez, J.; Pazos, I.; Martínez, M.C. Variability at the electron microscopy level in leaves of members of the genus Vitis. Sci. Hortic. 2011, 128, 228–238.
- Chitwood, D.H. The shapes of wine and table grape leaves: An ampelometric study inspired by the methods of Pierre Galet. Plants People Planet 2021, 3, 155–170.
- Monteiro, A.; Teixeira, G.; Lopes, C.M. Comparative leaf micromorphoanatomy of Vitis vinifera ssp. Vinífera (Vitaceae) red cultivars. Ciênc. Téc. Vitivinic. 2013, 28, 19–28.
- Pinto, T.M.; Anjos, M.R.; Martins, N.M.; Gomes-Laranjo, J.; Ferreira-Cardoso, J.; Peixoto, F. Structural analysis of Castanea sativa Mill. leaves from diferent regions in the treetop. Braz. Arch. Biol. Technol. 2011, 54, 117–124.
- Hogg, T.; Rebelo, J. Rumo Estratégico Para o Sector dos Vinhos do Porto e Douro, Síntese—Documento de Trabalho; IVDP, UTAD, Innovine and Wine: Vila Real, Portugal, 2017; ISBN 978-989-704-344-4.
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; Kumar, N.V.A.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947.
- Pinto, T.; Vilela, A. Healthy Drinks with Lovely Colors: Phenolic Compounds as Constituents of Functional Beverages. Beverages 2021, 7, 12.
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699.
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, drinks, and spice: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897.
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 2010, 31, 446–467.
- Domínguez-Avila, J.A.; Villa-Rodriguez, J.A.; Montiel-Herrera, M.; Pacheco-Ordaz, R.; Roopchand, D.E.; Venema, K.; González-Aguilar, G.A. Phenolic Compounds Promote Diversity of Gut Microbiota and Maintain Colonic Health. Dig. Dis. Sci. 2020.
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501.
- Zhao, X.; Wu, Y.; Liu, H.; Hu, N.; Zhang, Y.; Wang, S. Grape seed extract ameliorates PhIP-induced colonic injury by modulating gut microbiota, lipid metabolism, and NF-κB signaling pathway in rats. J. Funct. Foods 2021, 78, 1–12.
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528.
- Du, K.; McGill, M.R.; Xie, Y.; Bajt, M.L.; Jaeschke, H. Resveratrol Prevents Protein Nitration and Release of Endonucleases from Mitochondria During Acetaminophen Hepatotoxicity. Food Chem. Toxicol. 2015, 81, 62–70.
- Arts, I.C.; Hollman, P.C. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S.
- Peters, U. Does Tea Affect Cardiovascular Disease? A Meta-Analysis. Am. J. Epidemiol. 2001, 154, 495–503.
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol modulates pro-inflammatory NF-κB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. AGE 2010, 32, 197–208.
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.-P.; Nettleton, J.A.; Jacobs, D.R. Flavonoid Intake and Cardiovascular Disease Mortality: A Prospective Study in Postmenopausal Women. Am. J. Clin. Nutr. 2007, 85, 895–909.
- Hessami, A.; Shamshirian, A.; Heydari, K.; Pourali, F.; Alizadeh-Navaei, R.; Moosazadeh, M.; Abrotan, S.; Shojaie, L.; Sedighi, S.; Shamshirian, D.; et al. Cardiovascular diseases burden in COVID-19: Systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 46, 382–391.
- Matsushita, K.; Ding, N.; Kou, M.H.; Hu, X.; Chen, M.K.; Gao, Y.M.; Honda, Y.; Zhao, D.; Dowdy, D.; Mok, Y.; et al. The Relationship of COVID-19 Severity with Cardiovascular Disease and Its Traditional Risk Factors: A Systematic Review and Meta-Analysis. Glob. Heart 2020, 15, 64.
- Bae, S.; Kim, S.R.; Kim, M.N.; Shim, W.J.; Park, S.M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: A systematic review and meta-analysis. Heart 2021, 107, 373–380.
- Fathi, M.; Vakili, K.; Sayehmiri, F.; Mohamadkhani, A.; Hajiesmaeili, M.; Rezaei-Tavirani, M.; Eilami, O. The prognostic value of comorbidity for the severity of COVID-19: A systematic review and meta-analysis study. PLoS ONE 2021, 16, e0246190.
- Naeini, M.B.; Sahebi, M.; Nikbakht, F.; Jamshidi, Z.; Ahmadimanesh, M.; Hashemi, M.; Ramezani, J.; Miri, H.H.; Yazdian-Robati, R. A meta-meta-analysis: Evaluation of meta-analyses published in the effectiveness of cardiovascular comorbidities on the severity of COVID-19. Obes. Med. 2021, 22, 100323.
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613.
- Lin, S.C.; Ho, C.T.; Chuo, W.H.; Li, S.M.; Wang, T.T.; Lin, C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144.
- Yang, M.H.; Wei, J.L.; Huang, T.; Lei, L.P.; Shen, C.G.; Lai, J.Z.; Yang, M.; Liu, L.; Yang, Y.; Liu, G.S.; et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phyther. Res. 2020, 35, 1127–1129.
- Wahedi, H.M.; Ahmad, S.; Abbasi, S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2020, 39, 3225–3234.
- Giordo, R.; Zinellu, A.; Eid, A.H.; Pintus, G. Therapeutic Potential of Resveratrol in COVID-19-Associated Hemostatic Disorders. Molecules 2021, 26, 856.
- Rafe, T.; Shawon, P.A.; Salem, L.; Chowdhury, N.I.; Kabir, F.; Bin Zahur, S.M.; Akhter, R.; Noor, H.B.; Mohib, M.M.; Sagor, M.A.T. Preventive Role of Resveratrol Against Inflammatory Cytokines and Related Diseases. Curr. Pharm. Des. 2019, 25, 1345–1371.
- Giordo, R.; Nasrallah, G.K.; Al-Jamal, O.; Paliogiannis, P.; Pintus, G. Resveratrol Inhibits Oxidative Stress and Prevents Mitochon2drial Damage Induced by Zinc Oxide Nanoparticles in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2020, 21, 3838.
- Mittra, I.; de Souza, R.; Bhadade, R.; Madke, T.; Shankpal, P.D.; Joshi, M.; Qayyumi, B.; Bhattacharjee, A.; Gota, V.; Gupta, S.; et al. Resveratrol and Copper for treatment of severe COVID-19: An observational study (RESCU 002). medRxiv 2020.
- Emmanuel, R.D.; Lawrence, A.B.; Oluyomi, A.S. COVID 19: Resveratrol as a Potential Supplement to Mitigate the Cardiotoxicity Associated with Chloroquine and Hydroxychloroquine Treatment. Biointerface Res. Appl. Chem. 2021, 11, 11172–11186.
- Peynaud, E.; Ribéreau-Gayon, P. The grape. In The Biochemistry of Fruits and Their Products; Hulme, A.C., Ed.; Academic Press: London, UK, 1971; Volume 2.
- Ali, K.; Maltese, F.; Choi, Y.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378.
- Sat, I.G.; Sengul, M.; Keles, F. Use of Grape Leaves in Canned Food. Pak. J. Nutr. 2002, 1, 257–262.
- Radovanović, B.; Andjelkoví, M.; Radovanović, V.; Milenković-Andjelković, A.; Djekić, S. Polyphenols and Antioxidant Activity of Dierent Vinegrape Leaves. Zb. Rad. 2015, 20, 347–352.
- Balìk, J.; Kyseláková, M.; Vrchotová, N.; Triska, J.; Kumsta, M.; Veverka, J.; HÍc, P.; Totusek, J.; Lefnerová, D. Relations between polyphenols content and antioxidant activity in vine grapes and leaves. Czech J. Food Sci. 2008, 26, S25–S32.
- Lacerda, D.S.; Costa, P.C.; Funchal, C.; Dani, C.; Gomez, R. Benefits of Vine Leaf on Different Biological Systems. In Grape and Wine Biotechnology; IntechOpen: London, UK, 2016; pp. 125–143.
- Katalinic, V.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Teskera, A.; Konta, I.; Boban, M. Insight in the phenolic composition and antioxidative properties of Vitis vinifera leaves extracts. Croat. J. Food Sci. Technol. 2009, 1, 7–15.
- Monagas, M.; Hernández-Ledesma, B.; Gómez-Cordovés, C.; Bartolomé, B. Commercial dietary ingredients from Vitis vinifera L. leaves and grape skins: Antioxidant and chemical characterization. J. Agric. Food Chem. 2006, 54, 319–327.
- Valcárcel-Muñoz, M.J.; Guerrero-Chanivet, M.; García-Moreno, M.V.; Rodríguez-Dodero, M.C.; Guillén-Sánchez, D.A. Comparative Evaluation of Brandy de Jerez Aged in American Oak Barrels with Different Times of Use. Foods 2021, 10, 288.
- Lapuerta, M.; Rodríguez-Fernández, J.; Ramos, Á.; Donoso, D.; Canoira, L. WLTC and real-driving emissions for an autochthonous biofuel from wine-industry waste. Sci. Rep. 2021, 11, 7528.
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46.
- Rasines-Perea, Z.; Teissedre, P.L. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017, 22, 68–87.
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food by-products valorisation: Grape pomace and olive pomace (pâté) as sources of phenolic compounds and fiber for enrichment of tagliatelle pasta. Food Chem. 2021, 355, 129642.
- Sri Harsha, P.S.C.; Lavelli, V. Use of Grape Pomace Phenolics to Counteract Endogenous and Exogenous Formation of Advanced Glycation End-Products. Nutrients 2019, 11, 1917.
- Morales-Prieto, N.; Huertas-Abril, P.V.; López de Lerma, N.; Pacheco, I.L.; Pérez, J.; Peinado, R.; Abril, N. Pedro Ximenez sun-dried grape must: A dietary supplement for a healthy longevity. Food Funct. 2020, 11, 4387–4402.
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355.
- Rahimmalek, M.; Goli, S.A.H. Evaluation of six drying treatments with respect to essential oil yield, composition, and color characteristics of Thymys daenensis subsp. daenensis. Celak leaves. Ind. Crops Prod. 2013, 42, 613–619.
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149.
- Figueroa-Robles, A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Encapsulation of phenolic compounds with liposomal improvement in the cosmetic industry. Int. J. Pharm. 2021, 593, 120125.
- Li, W.; Li, Y.; Bi, J.; Ji, Q.; Zhao, X.; Zheng, Q.; Tan, S.; Gao, X. Effect of hot air drying on the polyphenol profile of Hongjv (Citrus reticulata Blanco, CV. Hongjv) peel: A multivariate analysis. J. Food Biochem. 2020, 44, e13174.
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594.
- Weggler, B.A.; Gruber, B.; Teehan, P.; Jaramillo, R.; Dorman, F.L. Chapter 5—Inlets and sampling. In Separation Science and Technology; Nicholas, H.S., Ed.; Academic Press: London, UK, 2020; Volume 12, pp. 141–203.
- Li, H.; Chen, B.; Nie, L.; Yao, S. Solvent effects on focused microwave-assisted extraction of polyphenolic acids from Eucommia ulmodies. Phytochem. Anal. 2004, 15, 306–312.
- Escobar-Avello, D.; Mardones, C.; Saéz, V.; Riquelme, S.; von Baer, D.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Pilot-plant scale extraction of phenolic compounds from grape canes: Comprehensive characterization by LC-ESI-LTQ-Orbitrap-MS. Food Res. Int. 2021, 143, 110265.
- Lončarić, A.; Lamas, J.P.; Guerra, E.; Kopjar, M.; Lores, M. Thermal stability of catechin and epicatechin upon disaccharides addition. Int. J. Food Sci. Technol. 2018, 53, 1195–1202.
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11.
- Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; Martínez de la Ossa, E.J. Green extraction of antioxidants from different varieties of red grape pomace. Molecules 2015, 20, 9686–9702.
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118.
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867.
- Montibeller, M.J.; Monteiro, P.L.; Stoll, L.; Tupuna-Yerovi, D.S.; Rodrigues, E.; Rodrigues, R.C.; Rios, A.O.; Manfroi, V. Improvement of enzymatic assisted extraction conditions on anthocyanin recovery from different varieties of V. vinifera and V. labrusca grape pomaces. Food Anal. Meth. 2019, 12, 2056–2068.
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109.
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound-assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47.
- Gerardi, C.; D’amico, L.; Migoni, D.; Santino, A.; Salomone, A.; Carluccio, M.A.; Giovinazzo, G. Strategies for Reuse of Skins Separated From Grape Pomace as Ingredient of Functional Beverages. Front. Bioeng. Biotechnol. 2020, 26, 645.
- Goulas, V.; Stavrou, K.; Michael, C.; Botsaris, G.; Barbouti, A. The Potential of Sun-Dried Grape Pomace as a Multi-Functional Ingredient for Herbal Infusion: Effects of Brewing Parameters on Composition and Bioactivity. Antioxidants 2021, 10, 586.
- Bekhit, A.E.-D.; Cheng, V.J.; McConnell, M.; Zhao, J.H.; Sedcole, R.; Harrison, R. Antioxidant activities, sensory and anti-influenza activity of grape skin tea infusion. Food Chem. 2011, 129, 837–845.
- HMPC (Herbal Medicinal Products Committee). Assessment report on Vitis vinifera L., folium. In European Medicines Agency; EMA/HMPC/464682/2016: London, UK, 2017; 44p.
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentao, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440.
- Rizzuti, A.; Caliandro, R.; Gallo, V.; Mastrorilli, P.; Chita, G.; Latronico, M. A combined approach for characterisation of fresh and brined vine leaves by X-ray powder diffraction, NMR spectroscopy and direct infusion high resolution mass spectrometry. Food Chem. 2013, 141, 1908–1915.
- Koşar, M.; Kupeli, E.; Malyer, H.; Uylasüer, V.; Turkben, V.C.; Basüer, K.H.C. Effect of brining on biological activity of leaves of Vitis vinifera L. (Cv. Sultani Cekirdeksiz) from Turkey. J. Agric. Food Chem. 2007, 55, 4596–4603.
- Deliorman-Orhan, D.; Orhan, N.; Özçelik, B.; Ergun, F. Biological activities of Vitis vinifera L. leaves. Turk. J. Biol. 2009, 33, 341–348.
- Ceyhan, N.; Keskin, D.; Zorlu, Z.; Ugur, A. In-vitro antimicrobial activities of different extracts of grapevine leaves (Vitis vinifera L.) from West Anatolia against some pathogenic microorganisms. J. Pure Appl. Microbiol. 2012, 6, 1303–1308.
- Fernandes, B.; Correia, A.C.; Cosme, F.; Nunes, F.M.; Jordão, A.M. Volatile components of vine leaves from two Portuguese grape varieties (Vitis vinifera L.), Touriga Nacional and Tinta Roriz, analysed by solid-phase microextraction. Nat. Prod. Res. 2015, 29, 37–45.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
11 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




