1. Introduction
Worldwide, gastric cancer ranks fifth in incidence and fourth in mortality, registering one million new cases and an estimated 769,000 deaths in 2020
[1]. There are two topographical subtypes, the cardia (upper stomach) and non-cardia (lower stomach), which have different risk, carcinogenesis, and epidemiologic factors. Incidence and mortality rates of non-cardia cancer have been decreasing over the last fifty years due to prevention (e.g.,
Helicobacter pylori infection and storage of foods), although a significant increase in the incidence of stomach cancer among young adults (aged < 50 years) was reported
[2].
Both gastric precancerous lesions and gastric cancer are associated with a spectrum of genetic and epigenetic abnormalities
[3][4][5]. These include genetic instabilities and abnormalities in oncogenes, tumor suppressor genes, growth factors, receptor tyrosine kinases, DNA repair genes, matrix degradation enzymes, cell-cycle regulators, and cell adhesion molecules. Somatic mutations may occur in proliferative and preinvasive lesions (e.g., mutations of CTNNB1 in fundic gland polyps, GNAS in pyloric gland adenomas, and TP53 in high-grade dysplasia and adenocarcinoma)
[6][7][8][9][10].
According to Lauren’s classification, gastric carcinoma is classified as intestinal-type and diffuse-type
[11]. Intestinal-type cancers show biological and epidemiological features that are different from those of diffuse-type cancers
[12]. These data suggest that tumors displaying different biological aspects are associated with a distinct clinical behavior.
Recently, new molecular classifications of gastric cancer were introduced. In 2014, based on key DNA defects and molecular abnormalities, The Cancer Genome Atlas Consortium (TCGA) divided gastric cancers into Epstein-Barr virus (EBV)-positive, microsatellite instability (MSI), gene stable (GS), and chromosome instability (CIN) types
[13]. TCGA typing is based on European and U.S. populations; however, the clinical characteristics of TCGA typing in the Asian population and its association with clinical parameters and prognosis remain unclear.
To prevent the development of gastric cancer, the diagnosis of precancerous lesions is crucial and should be encouraged, especially in countries where organized screening programs do not exist, and in areas with a high incidence of gastric cancer. In Japan, where organized screening programs are still active, more than 50% of gastric cancers are diagnosed in the early phase. Patients affected by early gastric cancers have a good prognosis, with overall survival rates close to 100% after 5 years of follow up and can be treated with radical endoscopic resection (ESD)
[14].
Another important aspect of early diagnosis and prevention is hereditary diffuse gastric cancer (HDGC), characterized by the prevalence of diffuse gastric cancer and lobular breast cancer. It is largely caused by inactivating germline mutations in the tumor suppressor gene CDH1, although pathogenic variants in CTNNA1 occur in a minority of families with HDGC.
Recent clinical practice guidelines for HDGC from the International Gastric Cancer Linkage Consortium (IGCLC), which recognizes the emerging evidence of variability in gastric cancer risk between families with HDGC, focus on the growing capability of endoscopic and histological surveillance in HDGC and increased experience of managing the long-term sequelae of total gastrectomy in young patients. Prophylactic total gastrectomy remains the recommended option for gastric cancer risk management in pathogenic CDH1 variant carriers. However, there is increasing confidence from the IGCLC that endoscopic surveillance in expert centers can be safely offered to patients who wish to postpone surgery or to those whose risk of developing gastric cancer is not well-defined
[15]. The precancerous stages of intestinal-type gastric cancer represent a complex process, part of which results in a transformation of the normal mucosa to an intestinal metaplastic mucosa through a series of lesions forming a continuum. This sequence of events may last for several years and is designated as Correa’s cascade of multistep gastric carcinogenesis
[16]. According to this scheme, the morphological changes observed fall into three categories: chronic inflammation of the gastric mucosa (chronic gastritis), mucosal loss of appropriate gastric glands (atrophy), and substitution of gastric epithelium with intestinal epithelium (intestinal metaplasia).
Through successive mutations, the gastric epithelial cells disappear and are replaced by cells with an intestinal phenotype, which, over time, gain autonomy, favoring the development of dysplastic changes (intraepithelial neoplasia) and carcinoma.
Correa’s cascade accounts for the carcinogenesis of intestinal-type gastric cancer. In this regard, the OLGA system provides a basis for predicting gastric cancer risks associated with atrophic gastritis and intestinal metaplasia, as well as guiding clinical surveillance
[16][17].
Intestinal-type adenocarcinomas are subclassified into three groups: foveolar, intestinal, and combined. These three groups have different expressions of mucins, have distinct cellular mucin phenotypes resulting from different genetic alterations, and are clinically relevant. Similarly, recent studies reported different patterns of mucins expression in preneoplastic lesions, suggesting biological specific pathways. Complete intestinal metaplasia shows goblet cells, absorptive enterocytes with a luminal brush border, and intestinal mucin (
MUC2) expression. In contrast, incomplete intestinal metaplasia displays goblet cells, absorptive cells without a brush border, and co-expression of intestinal (
MUC2) and gastric (
MUC5AC,
MUC6) mucins
[18].
In this review, we summarize the most recent knowledge of mucins expression in preneoplastic and neoplastic lesions of the stomach, with emphasis on their precise clinicopathologic and prognostic role.
Unlike the intestinal-type, the diffuse-type gastric cancer, according to Lauren, had never been studied for mucins expression before the recent paper by the European Chapter of the International Gastric Cancer Association
[19]. In this study, researchers found that mucins do not help in distinguishing signet ring cell from non-signet-ring cell gastric carcinoma. However, mucin stains expression helps identify different outcomes. Furthermore, outcomes and mucins expression seem to differ between Caucasian and Asian patients.
2.Early Gastric Cancer
The term early gastric cancer (EGC) and its definition as a carcinoma limited to the mucosa and/or submucosa, regardless of lymph-node status, was first proposed in 1971 and then included in the guidelines of the Japanese Gastric Cancer Association
[20].
This definition has been criticized, especially with the rise in endoscopic treatment for early lesions. Many studies were conducted, focusing on parameters that can be associated with adverse prognosis, treatment failure, or lymph node metastases in ECG, without reaching a definitive consensus. The main problem originates from the lack of clear criteria distinguishing ECG with excellent prognosis (>98% 5-year survival) from ECG with higher incidence of lymph node metastases and worse prognosis (70% 5-year survival).
The presence of lymph node metastases at diagnosis is due to predictive parameters, including growth patterns of infiltration of the submucosa, according to Kodama’s classification (
Table 1). Kodama’s PEN A type growth patterns are independent negative prognostic factors, identifying tumors with clinical behavior similar to that of advanced cancers, as we have published in a few previous studies
[21][22][23][24].
Table 1. Kodama’s classification.
| Kodama’s Types |
Description |
| Small mucosal |
|
| Mucosal (M) |
Intramucosal EGCs measuring less than 4 cm |
| Submucosal (SM) |
Intramucosal EGCs minimally invading submucosa measuring less than 4 cm |
| Super mucosal |
|
| Mucosal (M) |
Intramucosal EGCs measuring more than 4 cm |
| Submucosal (SM) |
Intramucosal EGCs minimally invading submucosa measuring more than 4 cm |
| Pen (penetrating) |
|
| A |
EGCs massively invading submucosa with nodular pattern measuring less than 4 cm |
| B |
EGCs massively invading submucosa with saw teeth pattern measuring less than 4 cm |
| Mixed |
Penetrating types (A or B) measuring more than 4 cm |
Many parameters can be implicated in the different prognoses of EGC subtypes, but they have not been thoroughly explored. In the 1980s, Inokuchi demonstrated the correlation between a different cell nuclear DNA distribution pattern and a malignancy in PEN A that is characterized by aneuploid and a high-ploidy DNA range, such as carcinoma
[25]. In recent studies, the aggressiveness of EGC correlated with a tumor microenvironment and genomic features (e.g.,
MUC1 expression and gastric carcinoma)
[26][27][28].
In 1999, Egashira et al.
[29] showed that the differentiated (DA) minute (<5 mm) adenocarcinomas with gastric phenotype differ morphologically and histogenetically from DA with intestinal phenotype, as the former lack intestinal metaplasia in the surrounding non-neoplastic mucosa. However, they demonstrated that as the tumor with gastric phenotype grows, intestinal metaplasia progresses, intestinal-type phenotypic expression appears, and then DA with gastric phenotype changes into DA with gastric-intestinal or intestinal phenotype.
In another study, the histologic conversion from differentiated type carcinoma (DC) to undifferentiated type carcinoma (UDC) seemed to occur mainly in gastrointestinal mucin phenotype (GIM-type) and gastric mucin phenotype (GM-type) tumors, depending on the size of the lesion. In other words, with an increase in tumor size, small DCs with GIM and GM phenotypes might change histologically into UDCs. Instead, DCs with intestinal mucin (IM) phenotypic expression rarely show histologic conversion
[25].
Regarding molecular alterations, Tsukashita, in 2001
[26], evaluated the histogenesis of gastric adenocarcinoma by
MUC gene expression in eighty intramucosal gland-forming tumors. These tumors were categorized in three groups (according to the Vienna classification): group A (low-grade adenoma/dysplasia), group B (high-grade adenoma/dysplasia), and group C (intramucosal carcinoma). There were different expressions of the
MUC genes: in group A, the lesions expressed intestinal markers and had a stable intestinal phenotype; whereas more than 50% of group B and C tumors expressed gastric markers, were unstable, and should have been considered de novo carcinomas.
Types II and III intestinal metaplasia tend to show mixed differentiation, including gastric- and intestinal-type cells, often expressing MUC2, MUC5AC, and MUC6 at the same time. Gastric mucosa surrounding minute adenocarcinomas also appears to be intestinalized and the metaplasia is of the incomplete type. The results of this study, in which MUC gene expression in incomplete form of intestinal metaplasia was similar to that in group B and C tumors, also support this hypothesis.
Moreover, despite the small number of cases, a recent article demonstrated the possibility to differentiate between Pen A and Pen B EGCs using an immunohistochemical stain for mucin
MUC6 (more expressed in Pen A tumors) and analyzing the copy number of GATA6 (more frequently amplified in Pen B types), introducing the possibility of distinguishing these two types of lesions in small pre-operatory biopsies
[28]. In this study, Molinari et al. analyzed 33 Pen A, 34 Pen B, and 20 T3N0 tumors (control group) and performed immunohistochemistry for mucins, copy number variation analysis of a gene panel, microsatellite instability (MSI), TP53 mutation, and loss of heterozygosity (LOH) analyses. The results showed that the Pen A subgroup was significantly characterized by
MUC6 overexpression (
p = 0.021). Otherwise, the Pen B type was significantly associated with the amplification of the GATA6 gene (
p = 0.002). A higher percentage of MSI tumors was observed in the T3N0 control group (
p = 0.002), but no significant differences between the two EGC subtypes were found. Finally, the TP53 gene analysis showed that 32.8% of Pen tumors had a mutation in exons 5–8 and 50.0% presented LOH. The co-occurrence of the TP53 mutation and LOH mainly characterized Pen A tumors (
p = 0.022).
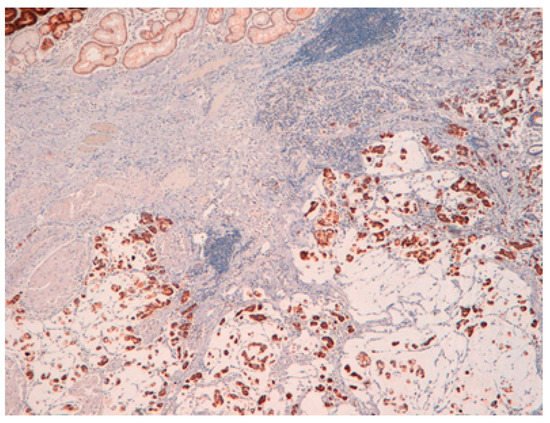
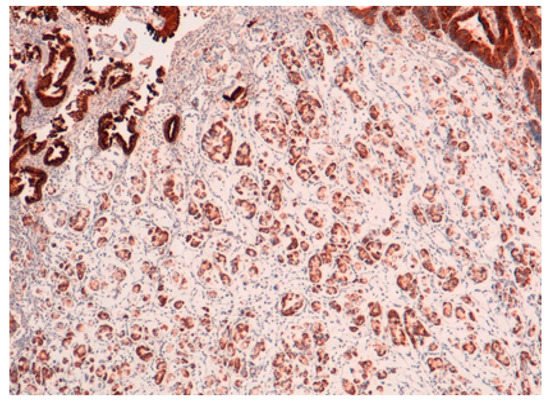
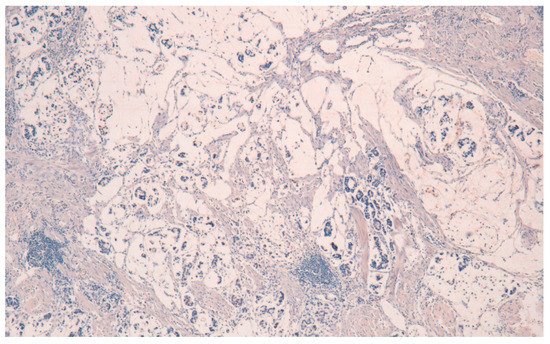
The result is that clinic-pathologic parameters, microsatellite status, and frequency of TP53 mutations do not seem to distinguish Pen subgroups. Conversely, the amplification of GATA6 is associated with Pen B tumors, and the overexpression of MUC6 and the TP53mut/LOH significantly characterizes Pen A lesions (Figure 1, Figure 2 and Figure 3).
Figure 1. Pen A EGC showing infiltration of the submucosa layers in nodular masses.
Figure 2. MUC6 positivity in Pen A ECG (50×).
Figure 3. MUC5AC negativity in Pen A ECG (50×).
All the photos are from the same lesion, were published with permission from Francesco Limarzi, and were not previously published in another journal.
Considering the signet ring cell carcinoma (SRC-GC) that falls into the group of diffuse-type gastric cancer and shows a better prognosis in its early phase compared to poorly cohesive intramucosal cancers without signet ring cells, 12 studies on histochemical mucins expression were published between 1977 and 2013, according to Lauren. The studies of Kubota, Akamatsu, and Tatematsu distinguished SRC-GC into several subtypes. Kubota et al.
[30] used Alcian Blue (AB), Alcian Blue-PAS (AB-PAS), and LNAase stain to classify 64 SRC-GC as type A (immature: PAS weak positive, AB negative, LNAase positive, small cell size, and high nuclear/cytoplasmic ratio), type B (intermediate: stronger PAS positivity, AB-negative or -weak-positive, LNAase-positive, and smaller nuclear/cytoplasmic ratio), or type C (mature: PAS-strong-positive, AB -positive, and eccentric nucleus).
Akamatsu et al.
[31] used five stains, AB-PAS, HID-AB, GOS, PA-SB-PH-PAS, and PCS to distinguish 31 SRC-GC into six subtypes: surface mucous cell-type, mucous neck cell-pyloric cell-type, goblet cell (small intestine)-type, goblet cell (large intestine)-type, microcyst-type, and unclassified.
Tatematsu et al.
[32][33] used PCS, GOS, and sialidase GOS with immunohistochemical stains for pepsinogen I and II to distinguish 127 SRC-GC as gastric phenotype, intestinal phenotype, or mixed gastrointestinal phenotype, with the gastric phenotype resulting in the most prevalent subtype.
These subclassifications of SRC-GC have not been validated in other studies.
In order to better define signet ring cell carcinoma and its prognosis compared to poorly cohesive gastric cancer without SRCs, Kerckhoffs et al.
[19], in an article which, to the best of our knowledge, is the largest study where all cancers were reclassified in a standardized manner according to WHO classification, compares Asian and Caucasian patients for the first time according to mucins expression, considering the relationship between mucins expression and patient outcome. The article shows no immunohistochemical mucins stain unique to SRC-GC. However, the mucins expression may be related to the quantity of SRCs within a given tumor, as the authors noticed a more frequent expression of mucins in poorly cohesive gastric cancer/diffuse gastric cancer containing > 10% SRCs. In their series, there are poorly cohesive cancers with ≥10% SRCs expressed more frequently:
MUC2,
MUC5AC, and ABPAS (
p < 0.001,
p = 0.004, and
p < 0.001, respectively). From a prognostic point of view, patients with
MUC2 positive SRC-GC or SRC-GC with (gastro)intestinal phenotype have the poorest outcome. Moreover, Caucasians with AB-positive GC or combined ABPAS-
MUC2-positive and
MUC5AC-negative have the poorest outcome (all
p = 0.002), whereas this association is not seen in Asian patients.