Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Agata Pietrzak | + 1715 word(s) | 1715 | 2021-08-05 04:36:43 | | | |
| 2 | Bruce Ren | -21 word(s) | 1694 | 2021-08-09 03:31:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pietrzak, A. [18F]FDG PET/CT Diagnosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/12890 (accessed on 12 January 2026).
Pietrzak A. [18F]FDG PET/CT Diagnosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/12890. Accessed January 12, 2026.
Pietrzak, Agata. "[18F]FDG PET/CT Diagnosis" Encyclopedia, https://encyclopedia.pub/entry/12890 (accessed January 12, 2026).
Pietrzak, A. (2021, August 06). [18F]FDG PET/CT Diagnosis. In Encyclopedia. https://encyclopedia.pub/entry/12890
Pietrzak, Agata. "[18F]FDG PET/CT Diagnosis." Encyclopedia. Web. 06 August, 2021.
Copy Citation
According to the international societies’ recommendations, the 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography/computed tomography ([18F]FDG PET/CT) technique should not be used as the method of choice in brain tumour diagnosis. Therefore, the brain region can be omitted during standard [18F]FDG PET/CT scanning. We performed comprehensive literature research and analysed results from 14,222 brain and torso [18F]FDG PET/CT studies collected in 2010–2020 to discuss whether the [18F]FDG PET/CT method may be useful in brain tumours detection.
brain tumour
18F-fluorodeoxyglucose
oncology
positron emission tomography
1. Introduction
Brain tumours are relatively rare malignancies, approximating 2% of all oncologic diseases. Currently, the incidence of primary and metastatic brain tumours seems to be especially increasing in highly developed countries, and oligometastatic brain disease seems to be especially concerning [1][2][3]. Malignant brain tumours are often deadly and involve several neurological and locomotory health ailments. Thus, both primary and metastatic brain tumour patients undergo treatment. Most often, therapy involves chemotherapy or chemoradiation rather than surgery. Histologic examination is not always available, due to the tumour location and the high post-surgical mortality risk among brain tumour patients. Therefore, prompt and complex diagnosis seems essential [1][3][4][5][6].
Central nervous system (CNS) disease diagnostic management involves mainly magnetic resonance imaging (MRI). However, the 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography/computed tomography ([18F]FDG PET/CT) study remains one of the most used imaging techniques in oncology. According to the international societies’ recommendations, the non-tumour-specific properties of the radiotracer [18F]FDG limit the specificity of the method in brain tumour detection and the CNS benign versus (vs.) malignant lesion differentiation. Therefore, the brain region is often omitted in the standard [18F]FDG PET/CT scanning protocol [7][8][9].
2. The Role of the [18F]FDG PET/CT Study in CNS Evaluation—Recommendations
Commonly used [18F]FDG PET/CT acquisition protocol ranges from the skull-base to mid-thigh [10][11][12][13][14][15][16][17][18]. Boellard et al. described the brain and torso [18F]FDG PET/CT [11] study protocol as a modification of the standard examination. The authors [10][11][12][13][14][15][16][17][18] discussed the potential utilities of [18F]FDG PET/CT imaging in brain disease diagnosis, including non-invasive staging for therapy planning. The main limitation of the [18F]FDG PET/CT study is the non-tumour-specific properties of the radiotracer [18F]FDG. According to the IAEA, EANM, and SNMMI recommendations [10][11][12][13][14][15][16], a high glucose uptake within the grey matter significantly decreases the specificity of the study in detecting small lesions within the CNS and distinguishing benign (i.e., inflammation) and malignant tumours. Nevertheless, IAEA [10] mentions the delayed PET/CT imaging at 4–6 h p.i. of [18F]FDG as improving the specificity of the method by increasing the tumour-to-background ratio. However, we did not mention delayed protocol in this study (Table 1).
Table 1. [18F]FDG PET/CT in brain tumour diagnosis—international societies’ recommendations.
| Source of Recommendations | Applications 4 | Limitations 5 |
|---|---|---|
| EANM 1 [11][12][15][16] |
Non-invasive tumour grading | Low specificity in metastatic brain tumour evaluation |
| SNMMI 2 [13][14] |
Benign and malignant brain tumour detection | Limited ability to assess small brain tumours |
| IAEA 3 [10] |
Primary and metastatic brain tumour detection | Low specificity in distinguishing benign and malignant brain lesions |
According to the authors [18][19][20][21][22][23], the PET-dedicated radiopharmaceuticals of choice in brain lesion diagnosis are [11C]-methionine ([11C]MET), [18F]-fluoroethyltyrosine ([18F]FET), [18F]-3′-deoxy-3′-fluorothymidine ([18F]FLT), and [18F]-dihydroxyphenylalanine ([18F]DOPA). Jung et al. [18] recommend using [11C]MET, [18F]FLT, [18F]DOPA to improve the sensitivity and specificity of the PET/CT method in low-grade glioma imaging. Moreover, [18F]DOPA has been recognised as particularly useful in remnant brain tumour diagnosis in patients who underwent surgery or both tumour resection and radiotherapy [19][20][21][22]. Authors [18][19][20][21][22] mention a high [18F]FDG uptake within the grey matter, as well as the limited ability of the [18F]FDG PET/CT method in benign vs. malignant CNS lesion differential diagnosis. According to the literature [18][19][20][21][22], the most suitable radiopharmaceuticals for brain tumour diagnosis are cell proliferation markers.
3. Original Database—Brain and Torso [18F]FDG PET/CT Study
3.1. Epidemiology
We examined 14,222 patients using the brain and torso [18F]FDG PET/CT. We found suspicious brain lesions in 155 patients. The group consisted of 96 women and 59 men; mean age ± standard deviation (S.D.) was 60 ± 12 years old (y.o.), age range: 26–84 y.o. Among women, the mean age ± S.D. was 60 ± 12 y.o., range: 26–84 y.o.; men: 61 ± 13 y.o., range: 26–84 y.o.
The benign lesion group consisted of 15 women (mean age ± S.D.: 63 ± 13 y.o., range: 35–84 y.o.) and 9 men (mean age ± S.D.: 60 ± 13 y.o., range: 40–83 y.o.). The primary brain tumour group consisted of 16 women (mean age ± S.D.: 64 ± 11 y.o., range: 46–81 y.o.) and 12 men (mean age ± S.D.: 63 ± 17 y.o., range: 26–84 y.o.). The metastatic foci group consisted of 65 women (mean age ± S.D.: 58 ± 12 y.o., range: 30–78 y.o.) and 38 men (mean age ± S.D.: 61 ± 11 y.o., range: 39–81 y.o.). In our study, we observed the highest brain finding incidence among women over the age of 60 y.o.
3.2. Benign Lesions
We evaluated 24 benign lesions in the brain region: 22 arachnoid cysts, 1 adenoma, and 1 unspecified vascular malformation. In one patient, the arachnoid cyst was one of a few brain foci (renal cancer metastasis). Arachnoid cysts determined 87.5% of all detected benign lesions and occurred in 13.5% of the examined 155 patients.
We detected benign brain lesions in patients diagnosed with breast cancer (five patients), as well as colorectal (four), cervical (three), ovarian (two), adrenal (one), adrenal (one) and renal (one), prostate (one), testicular (one), liver (one; hepatocellular cancer; HCC), and lung (one) cancer. In two subjects, the indications to perform the brain and torso [18F]FDG PET/CT scanning included malignant melanoma staging.
In this study, we observed a relatively low glucose metabolism activity within the benign brain lesions. The mean SUVmax ± S.D. was 1.0 ± 0.2, the median (Me) was 1.0, range: 1.0–2.0 (CI95 = (1.1; 1.3)). The variables were normally distributed (p = 0.8). Thus, the benign lesions group was homogenous, considering the metabolic activity of the observed lesions. Figure 3 shows the arachnoid cyst.
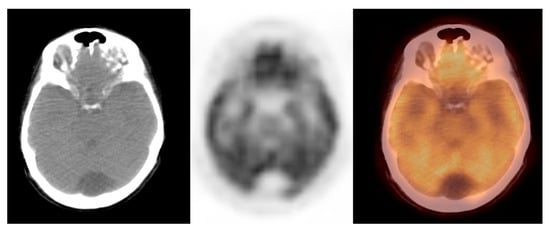
Figure 3. Incidental finding of a hypodense and photopaenic area in the posterior part of the brain in the middle cranial fossa, consistent with arachnoid cyst, in a patient with cervical cancer (source: original figure). Description: axial view of the brain and torso [18F]FDG PET/CT over the middle cranial fossa—low-dose CT (left-hand side image), PET (middle image), and PET/CT fusion (right-hand side image).
3.3. Primary Brain Tumours
We incidentally detected 28 primary brain tumours (18% of primary tumours and 21% of all malignant lesions). In this group, we found 10 gliomas, 8 pituitary gland tumours, 4 malignant meningiomas, 4 cerebelli primaries, 1 brain lymphoma, and 1 base of the skull tumour. In some of the examined patients, we observed multiple lesions (up to six foci). Gliomas determined nearly 36% of all brain primaries.
The primary brain lesions occurred in patients [18F]FDG PET/CT diagnosed with cancer of unknown primary (CUP syndrome; 18 patients), as well as colorectal (three patients), breast (one), cervical (one), oesophagal (one), lung (one), prostate (one), and uterine (one) cancer. In one patient, the primary disease was malignant melanoma.
We observed widely ranged glucose metabolism activity levels within the primary brain tumour group due to the presence of both non-[18F]FDG-avid and low- and high-grade tumours in the datasets. The mean SUVmax ± S.D. was 9.2 ± 4.7, the Me was 9.0, range: 1.2–25.0 (CI95 = (7.3; 11.0)). Figure 4 and Figure 5 show the primary brain tumours.
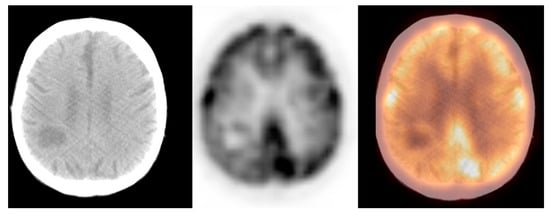
Figure 4. Incidental finding of a hypodense and photopaenic area in the right parietal lobe, consistent with a primary brain tumour, in a patient with larynx cancer (source: original figure). Description: axial view of the brain and torso [18F]FDG PET/CT over the middle cranial fossa—low-dose CT (left-hand side image), PET (middle image), and PET/CT fusion (right-hand side image).
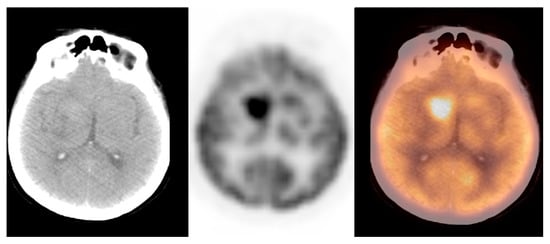
Figure 5. A hyperdense and hypermetabolic mass in the right thalamus region, consistent with a primary brain tumour (glioblastoma), in a patient with breast cancer (source: original figure). Description: axial view of the brain and torso [18F]FDG PET/CT over the middle cranial fossa—low-dose CT (left-hand side image), PET (middle image), and PET/CT fusion (right-hand side image).
3.4. Metastatic Foci
We detected 103 metastatic brain foci in 27 breast cancer patients (in one case, simultaneous breast and lung cancer), 20 lung cancer subjects (in one case, concurrent lung and colorectal cancer), malignant melanoma (25), colorectal cancer (nine), ovarian (four), renal (three), gastric (three), prostate (two), uterine (two), urinary bladder cancer (one), thyroid (two), and pancreas tumour (one), as well as Hodgkin’s (one) and non-Hodgkin’s lymphoma (three).
The oligometastatic brain disease (up to eight foci) occurred in nearly 70% of the metastatic foci database. Accordingly, with the patients’ medical records, in 45% of the group (46 among 103 cases), none other than brain lesions were detected. In approximately 10%, brain foci were one of the few observed lesions. In 35%, we found significant primary disease spread with multiple distant tumours with advanced lymph node involvement.
The metastatic foci group consisted of the most metabolically active malignant brain lesions. The mean SUVmax ± S.D. was 12.4 ± 5.6, the Me was 12.0, and the SUVmax range was 4.0–33.0 (CI95 = (11.3; 13.5)). The SUVmax distribution significantly differed from Gaussian, with p < 0.001. We observed the highest SUVmax levels within the lung cancer and the malignant melanoma metastases. In this study, we observed the highest glucose metabolism activity in the metastatic foci group. Figure 6 shows the metastatic brain lesion.
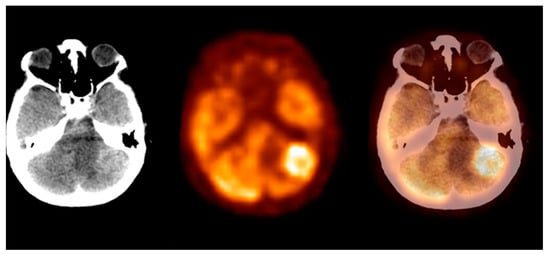
Figure 6. Incidental finding of a left lobe cerebellum abnormal mass, consistent with unknown and clinically silent lung cancer solitary metastatic lesion (source: original figure). Description: axial view of the brain and torso [18F]FDG PET/CT over the cerebellum—low-dose CT (left-hand side image), PET (middle image), and PET/CT fusion (right-hand side image).
4. Summary
Performing brain and torso [18F]FDG PET/CT examination resulted in detecting clinically silent malignant brain lesions in 155 patients diagnosed with different oncological diseases. The most numerous group was the metastatic foci, characterised by the highest mean SUVmax levels. When compared with the primary brain lesions, the distant tumour’s SUVmax level was significantly higher, with p = 0.003 (Mann–Whitney’s U-test). However, the differences in sample size limit the reliability of this comparison and do not allow the performing of benign and malignant tumour differential diagnosis analysis. Table 2 shows the SUVmax measurements.
Table 2. SUVmax: benign lesions, primary brain tumours, metastatic foci (source: original data).
| Group/Parameter | SUVmax 1 ± S.D. 2 | SUVmax Median | SUVmax Range | CI95 3 |
|---|---|---|---|---|
| Benign lesions | 1.0 ± 0.2 | 1.0 | 1.0–2.0 | [1.1; 1.3] |
| Primary brain tumours | 9.2 ± 4.7 | 9.0 | 1.2–25.0 | [7.3; 11.0] |
| Metastatic foci | 12.4 ± 5.6 | 12.0 | 4.0–33.0 | [11.3; 13.5] |
References
- Stewart, B.W.; Wild, C.P. Chapter 5.16. In World Cancer Report 2014, 1st ed.; Kleihues, P., Barnholtz-Sloan, J., Ohgaki, H., Eds.; WHO Press: Geneva, Switzerland, 2015; pp. 511–520.
- Nieder, C.; Hintz, M.; Popp, I.; Bilger, A.; Grosu, A.L. Long-term survival results after treatment for oligometastatic brain disease. Rep. Pract. Oncol. Radiother. 2020, 25, 307–311.
- National Brain Tumor Society. Available online: https://braintumor.org/brain-tumor-information/ (accessed on 1 April 2021).
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520.
- Pope, W.B. Brain metastases: Neuroimaging. Handb. Clin. Neurol. 2018, 149, 89–112.
- Gholamrezanezhad, A.; Shooli, H.; Jokar, N.; Nemati, R.; Assadi, M. Radioimmunotherapy (RIT) in Brain Tumors. Nucl. Med. Mol. Imaging 2019, 53, 374–381.
- Houston, S.F.; Abdelmalik, A.G.; Nguyen, N.C.; Farghaly, H.R.; Osman, M.M. Whole-body 18F-FDG PET/CT: The need for a standardized field of view—a referring-physician aid. J. Nucl. Med. Technol. 2010, 38, 123–127.
- Tasdemir, B.; Dostbil, Z.; Inal, A.; Unal, K.; Yildirim, S.; Simsek, F.S. Evaluation of clinical contributions provided by addition of the brain, calvarium, and scalp to the limited whole body imaging area in FDG-PET/CT tumor imaging. Biomed. Res. Int. 2014, 2014, 129683–129688.
- Fonti, R.; Pellegrino, S.; Mainolfi, C.G.; Matano, E.; Del Vecchio, S. Brain Metastases Unresponsive to Immunotherapy Detected by 18F-FDG-PET/CT in a Patient with Melanoma. Diagnostics 2020, 17, 410.
- International Atomic Energy Agency. Standard Operating Procedures for PET/CT: A Practical Approach for Use in Adult Oncology; IAEA Human Health Series No. 26; IAEA: Vienna, Austria, 2013.
- Boellard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354.
- Varrone, A.; Asenbaum, S.; Borght, T.V.; Booij, J.; Nobili, F.; Någren, K.; Darcourt, J.; Kapucu, Ö.L.; Tatsch, K.; Bartenstein, P.; et al. EANM procedure guidelines for PET brain imaging using 18F-FDG, version 2. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 2103–2110.
- Delbeke, D.; Coleman, R.E.; Guiberteau, M.J.; Brown, M.L.; Royal, H.D.; Siegel, B.A.; Townsend, D.W.; Berland, L.L.; Parker, J.A.; Hubner, K.; et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J. Nucl. Med. 2006, 47, 885–895, Erratum in J. Nucl. Med. 2006, 47, 903.
- Waxman, A.D.; Herholz, K.; Lewis, D.H.; Herscovitch, P.; Minoshima, S.; Ichise, M.; Drzezga, A.E.; Devous, M.D., Sr.; Mountz, J.M. Society of Nuclear Medicine Procedure Guideline for FDG PET Brain Imaging Version 1.0; Society of Nuclear Medicine: Reston, VA, USA, 2009.
- Testanera, G.; Pepe, G. Imaging in Oncological Brain Diseases: PET/CT. In A Technologist′s Guide-Brain Imaging, 1st ed.; Costa, P.F., Santos, A., Vidovič, B., Eds.; EANM: Vienna, Austria, 2015; pp. 33–53.
- Socan, A. Tracers for Brain Imaging. In A Technologist′s Guide-Brain Imaging, 1st ed.; Costa, P.F., Santos, A., Vidovič, B., Eds.; EANM: Vienna, Austria, 2015; pp. 12–25.
- Galldiks, N.; Langen, K.J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol. 2019, 21, 585–595.
- Jung, J.H.; Ahn, B.C. Current Radiopharmaceuticals for Positron Emission Tomography of Brain Tumors. Brain. Tumor. Res. Treat. 2018, 6, 47–53.
- Sharma, P.; Mukherjee, A. Newer positron emission tomography radiopharmaceuticals for radiotherapy planning: An overview. Ann. Transl. Med. 2016, 4, 53–60.
- Calabria, F.; Cascini, G.L. Current status of 18F-DOPA PET imaging in the detection of brain tumor recurrence. Hell. J. Nucl. Med. 2015, 18, 152–156.
- Calabria, F.; Chiaravalloti, A.; Di Pietro, B.; Grasso, C.; Schillaci, O. Molecular imaging of brain tumors with 18F-DOPA PET and PET/CT. Nucl. Med. Commun. 2012, 33, 563–570.
- Chen, W.; Silverman, D.H.; Delaloye, S.; Czernin, J.; Kamdar, N.; Pope, W.; Satyamurthy, N.; Schiepers, C.; Cloughesy, T. 18F-FDOPA PET imaging of brain tumors: Comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J. Nucl. Med. 2006, 47, 904–911.
- Treglia, G.; Muoio, B.; Trevisi, G.; Mattoli, M.V.; Albano, D.; Bertagna, F.; Giovanella, L. Diagnostic Performance and Prognostic Value of PET/CT with Different Tracers for Brain Tumors: A Systematic Review of Published Meta-Analyses. Int. J. Mol. Sci. 2019, 20, 4669–4685.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




