
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Corneliu Ovidiu Vrancianu | + 8209 word(s) | 8209 | 2020-06-23 10:15:43 | | | |
| 2 | Corneliu Ovidiu Vrancianu | + 1 word(s) | 8210 | 2020-06-28 06:38:39 | | | | |
| 3 | Catherine Yang | + 1 word(s) | 8210 | 2020-06-28 12:16:51 | | | | |
| 4 | Catherine Yang | -3709 word(s) | 4501 | 2020-07-02 05:06:03 | | | | |
| 5 | Catherine Yang | -28 word(s) | 4473 | 2020-10-26 12:03:05 | | |
Video Upload Options
Antibiotic resistance is one of the biggest challenges for the clinical sector and industry, environment and societal development. One of the most important pathogens responsible for severe nosocomial infections is Acinetobacter baumannii, a Gram-negative bacterium from the Moraxellaceae family, due to its various resistance mechanisms. The enormous adaptive capacity of A. baumannii and the acquisition and transfer of antibiotic resistance determinants contribute to the ineffectiveness of most current therapeutic strategies, including last-line or combined antibiotic therapy. In this review, we will present the current progress in developing innovative strategies for combating multidrug-resistant A. baumannii (MDRAB) infections.
1. Introduction
In recent years, the resistance phenomenon was encountered in most common bacterial strains causing infections, associated with an increased risk of morbidity, mortality, high treatment costs and long periods of hospitalization. One of the ESKCAPE pathogens responsible for nosocomial and community-acquired infections is Acinetobacter baumannii, a Gram-negative, non-motile, non-fermentative and non-sporulated bacterium from the Moraxellaceae family [1]. Two of the many reasons for the success of MDRAB strains are the association with chronic nosocomial infections and their unique ability to survive in extreme environmental conditions. Its reputation is mainly due to its association with severe infections caused to the US military during the wars in Afghanistan and Iraq, which is why it has been called “Iraqibacter” [2]. A. baumannii causes various infections, including pneumonia, urinary tract infections, skin and soft tissue infections or nosocomial meningitis [3]. Due to its extended resistome and virolome, evasion of the host's immune effectors, ability to grow in biofilms, to survive in extreme environmental conditions, and to switch to latent growth forms with a minimal metabolic rate, the treatment options are limited, rendering A. baumannii one of the most critical and fearful pathogens [4][5].
In this review, we will present an update regarding the perspectives of new therapeutic strategies efficient against MDRAB. In addition, the discussion section will present the main challenges of therapeutic strategies and the need for further studies in response to existing limitations.
2. Innovative Strategies for Treatment of A. baumannii Infections
Studies conducted in recent years highlight the unique involvement of A. baumannii strains in increasing the severity of nosocomial infections and, implicitly, their associated morbidity and mortality rates. Given that A. baumannii strains are resistant to almost all antibiotics used [6], the research direction must be in line with the “post-antibiotic era”, emphasizing the development of innovative strategies to control MDRAB spreading. Next, we will present the most innovative therapies, such as phage therapy, new antimicrobial peptides, and CRISPR Cas system (Clustered regularly interspaced short palindromic repeats) developed to prevent the spread of MDRAB strains.
2.1. Bacteriophages Therapy
Bacteriophages are viral parasites able to infect bacteria by recognizing surface receptors, injecting their genetic material into the host and replicating using the host cellular machinery [7]. Phages exhibit ecological and genetic effects on bacteria at the population level, and these effects can impact plasmid stability [8][9]. Phages may enhance the persistence of ARGs as an adaptation strategy to restrictive environmental conditions, e.g., wastewater aggressively treated using UV, temperature or pH. However, genetically modified phages could be used to increase antibiotic susceptibility of resistant strains. The alarming increase in the resistance rates has also led to the revival of phage therapy to increase the susceptibility level of bacteria by eliminating resistance and virulence markers [10]. In addition, research has shown that phage therapy has a high potential to represent an effective and safe treatment against MDRAB strains [12][13]. A high number of experiments were performed both in vitro and in vivo, the main results being summarized in Table 1.
Table 1. Bacteriophages therapy against A. baumannii strains.
|
Phages |
Family |
Isolation Source |
Type of Study |
Number of Tested Strains |
% of Susceptible Strains |
Animal Model Application |
References |
|
økm18p |
Corticoviridae |
hospital sewage |
in vitro |
34 MDR, 16 of those XDRAB |
44.1% |
NA |
[14] |
|
Acibel004 |
Myoviridae |
wastewater sample |
in vitro |
34 MDR |
82.3% |
NA |
[15] |
|
Acibel007 |
Podoviridae |
wastewater sample |
in vitro |
34 MDR |
82.3% |
NA |
|
|
IsfAB78 |
Myoviridae |
water sample |
in vitro |
43 MDR |
27.9% |
NA |
[16] |
|
IsfAB39 |
Podoviridae |
water sample |
in vitro |
43 MDR |
25.5% |
NA |
|
|
vB_AbaS_Loki |
Siphoviridae |
sludge |
in vitro |
34 |
5.8% |
NA |
[17] |
|
Petty phage |
Podoviridae |
sewage |
in vitro |
40, 25 of those MDR |
10% |
NA |
[18] |
|
SH-Ab 15599 |
Myoviridae |
sewage |
in vitro |
48 CRAB |
27% |
NA |
[19] |
|
SH-Ab15708 |
Myoviridae |
sewage |
in vitro |
48 CRAB |
29.1% |
NA |
|
|
SH-Ab15497 |
Siphoviridae |
sewage |
in vitro |
48 CRAB |
29.1% |
NA |
|
|
SH-Ab15519 |
Podoviridae |
sewage |
in vivo |
48 CRAB |
16.6% |
Mouse model—lung infection; 90% survival rate |
|
|
vBGEC_AbM-G7was (phiG7) |
Myoviridae |
sewage |
in vivo |
200 |
68% |
Rats wound model; 100% survival rate |
[20] |
|
Abp1 |
Moraxelaceae |
sewage |
in vitro |
20 |
NA |
Hella cells infection protection assay; 100% protection and survival rate of Hella cells. |
[21] |
|
in vivo |
20 |
|
Mouse local and systemic infection model; 100% survival rate. |
||||
|
PB AB08 |
Myoviridae |
Bacteriophage Bank of Korea |
in vivo |
14 MDR |
35.7% |
Mice model—intranasal phage cocktail; 35% survival rate |
[22] |
|
PBAB25 |
Myoviridae |
Bacteriophage Bank of Korea |
in vivo |
14 MDR |
7.1% |
Mice model—ntranasal phage cocktail; 35% survival rate. |
|
|
WCHABP1 |
Myoviridae |
hospital sewage |
in vivo |
2 CRAB |
NA |
Galleria mellonela infection model; 75% survival rate after phage administration |
[23] |
|
WCHABP12 |
Myoviridae |
hospital sewage |
in vivo |
NA |
|||
|
PD-6A3 |
Podoviridae |
sewage |
in vivo |
552 MDR |
32.4% |
Sepsis mouse model; intraperitoneal administration; endolysin therapy, endolysin + phage therapy, phage therapy and phage cocktail; 70%, 70%, 60% and 50% survival rate. |
[24] |
|
Bϕ-R2096 |
Myoviridae |
hospital sewage |
in vivo |
20 CRAB |
NA |
Galleria mellonella infection model; 80% and 50% survival rate at 96 and 48 h. |
[25] |
|
in vivo |
NA |
Mouse model acute pneumonia; 100%, 60% and 30% survival rate at day 12, with MOI 10, 1 and 0.1 |
|||||
|
AB3P1 |
NA |
sewage, farm soil, feces of sheep, chicken litter, swab for surgical lounge. |
in vivo |
23 |
78.2% |
Mice model; intraperitoneal administration of AB3 phages; 100% survival rate; |
[26] |
NA, not applicable; MOI = multiplicity of infection.
Bacteriophage therapy represents a promising tool in fighting MDR A. baumannii strains. Analyzation of the data summarized in Table 1 highlights that both in vitro and in vivo studies demonstrate the high efficiency, increasing the survival rate of organisms infected with A. baumannii strains. Based on the results obtained on animal models in the last ten years, numerous studies have focused on understanding the effectiveness of this therapy against chronic infections in the hospital units, as revealed by different clinical trials [10][27][28][29][30]. Schooley et al. have used phagotherapy in a patient with necrotic pancreatitis caused by an MDRAB strain [31].
Contrary to these studies, there are other reports of the inefficiency of phages in treating bacterial infections [32][33], which suggests that the clinical use of phages requires standardization. One of the most significant challenges in phage therapy is the resistance of bacterial strains to phage action [34][35][36]. In bacterial communities, resistance is a dynamic process when the antibacterial agent is biologic, as is the case with phages. On the other hand, phages exert a selective competition on bacteria. This two-way interaction causes a co-evolution that results in bacteria acquiring resistance mechanisms that can block the cycle of lytic infection [34][35]. Bacteria can acquire resistance to phages following cellular surface changes represented by point mutations in phage binding receptors [37]. Another mechanism of resistance is the outer membrane vesicles to which phages can bind due to surface structures, similar to those of parental cells. Binding of phages to these vesicles during invasion decreases the likelihood of cell infection [34]. In addition, a significant impact has the restriction–modification systems, the most common defense mechanisms in bacteria that can degrade foreign DNA, including double-stranded DNA phages [38]. Another concern in the use of phage therapy is the lack of standardization of phage preparation methods. An incomplete purification of host bacterial phages can lead to an unwanted transfer of bacterial toxins such as endotoxins or exotoxins [39]. Particular attention should be paid to combination therapy with phages and lysins. Once the dose needed to increase antimicrobial action has been determined, the mechanisms of action and elimination from the body must be established [40]. Stimulation by the phage of the immune response and adaptive immune systems, as well as their presence in the bloodstream, may influence the effectiveness of phage therapy [39].
Further studies are required to understand phage biology clearly and to better control clinical trials to standardize phagotherapy.
2.2. Antimicrobial Peptides (AMP)
Antimicrobial peptides may represent an alternative to antibiotics in the control of MDRAB strains spread. AMP is a class of compounds widespread in the living world as part of the innate immunity, acting as a primary barrier against infectious agents such as viruses, bacteria and fungi [41][42]. AMPs also play an essential role in regulating immune processes such as activating and recruiting immune system cells, angiogenesis and inflammation [43]. AMPs are amphipathic molecules with a positive electric charge, having a length of about 11–50 amino acid residues [43][44]. The main mechanisms of antimicrobial action of AMPs are the ability to cause cell membrane and cell wall damage, the inhibition of protein synthesis, nucleic acids and the induction of apoptosis and necrosis [45]. Due to these properties, AMPs have been considered an alternative to the use of antibiotics for limiting the spread and decreasing the infection rate and mortality control measures of nosocomial infections.
To be considered for therapy, AMPs must have a broad spectrum of action, high specificity and low cytotoxicity levels to mammalian cells [46]. The primary limitations that hinder the approval of systemic use of AMPs are sensitivity to enzymatic digestion and high toxicity, which is why most AMPs are applied topically and not orally or intravenously [47][48]. It has also been observed that certain physiological conditions, such as high concentrations of salts and serum components, can exert adverse effects on AMPs [49]. Compared to the conventional use of antibiotics, production costs for AMPs are much higher, which is why research is moving towards peptides as short as possible with stable properties [50]. Currently, research is aimed at developing technologies to improve the efficiency of AMPs in vivo, especially in terms of increasing the specificity against the infectious agents, decreasing cytotoxicity to mammalian cells, increasing stability and lowering production costs. The newest AMPs studied to elucidate their therapeutic efficacy against A. baumannii strains are summarised in table 2.
Table 2. Antimicrobial Peptides (AMPs) with antimicrobial activity against A. baumannii.
|
Organism |
AMP |
Type of Study |
Animal Model |
Main Results |
References |
|
NA |
ZY4 cathelicidin-BF-15 derived |
in vitro; in vivo |
mouse septicemia infection model |
Antibacterial activity in plasma; biofilm inhibition; kills persister cells; inhibition of infection and inflammation in vivo |
[51] |
|
NA |
epsilon-poly L-lysine (EPL)-catechol |
in vitro; in vivo |
mouse burn wounds infection model |
Reducing bacterial burden in vivo |
[52] |
|
Vespa affinis |
mastoparan-AF |
in vitro |
NA |
Potent antimicrobial activity |
[53] |
|
NA |
chex1-Arg20 amide (ARV-1502) |
in vivo |
Mouse infection model |
Reduction of bacterial load |
[54] |
|
NA |
α-helical -26 AMP residues |
in vitro |
NA |
Great antimicrobial activity |
[55] |
|
Delftia spp. |
delfibactin A |
in vitro |
NA |
Great inhibitory effects |
[56] |
|
Capra hircus |
mini-ChBac7.5Nα |
in vitro |
NA |
Significant antimicrobial activity; induce membrane damages; |
[57] |
|
Hybrid striped bass Morone saxatilis × M. chrysops |
I16 K-piscidine-1 analog |
in vitro; in vivo |
Sepsis mouse model |
Strong bactericidal activity; high survival rate of infected mice; |
[58] |
|
Musca domestica |
cecropin-4 |
in vitro |
NA |
Great bactericidal activity against MRAB and PRAB; inhibits biofilm formation |
[59] |
|
NA |
Ω17 and Ω76 family peptides |
in vitro; in vivo |
Mouse peritoneal infection model |
Disrupt bacterial membranes; induce small-molecule leakage; rapid bactericidal activity; |
[60] |
|
NA |
ceragenins (AMP synthetic mimics) |
in vitro |
NA |
Antibiofilm activity; inhibitory effects |
[61] |
|
Medicago truncatula |
nodule-specific cysteine-rich (NCR) peptide and its derivatives |
in vitro |
NA |
Potent killer of pathogenic bacteria |
[62] |
|
NA |
TAT-RasGAP317−326 anticancer peptide |
in vitro; in vivo |
Mousel model of lethal peritonitis |
Growth inhibition effects; broad-spectrum antimicrobial activity; great efficacy in vivo |
[63] |
|
NA |
WLBU2-cationic amphipathic peptide |
in vitro |
NA |
Eradicating bacterial biofilms; |
[64] |
|
Myxine glutinosa L. |
myxinidin 2; myxinidin 3 |
in vitro, |
Mouse skin wounds infection model |
Antibiofilm activity; anti-inflammatory activity; enhance wound healing; |
[65] |
|
Hepatitis B virus |
D-150–177C, HBcARD derivative peptide |
in vivo |
Mouse sepsis infection model |
Strong bactericidal activity; 90% of mice protected from death; |
[66] |
|
Pisum sativum |
nuripep 1653 |
in vitro |
NA |
Significant antimicrobial activity; |
[67] |
|
Cimex lectularius (bedbug) |
CL defensin |
in vitro |
NA |
Inducing membrane depolarization and pore forming; bactericidal action |
[68] |
|
Bungarus fasciatus |
cathelicidin—BF derivate (Cath-A) |
in vitro |
NA |
Bacterial growth inhibition |
[69] |
|
Lucilia sericata |
LS-sarcotoxin and LS-stomoxyn |
in vitro; in vivo |
Mouse model infection |
Strong activity against GRAM-NEGATIVE; |
[70] |
|
Leiurus quinquestriatus |
venom cocktail proteins |
in vitro |
NA |
Broad-spectrum antimicrobial activity; growth inhibition; |
[71] |
|
Myrmecia pilosula |
Δ-Myrtoxin-Mp1a (Mp1a) heterodimeric peptide |
in vitro; in vivo |
Mouse model |
Antibacterial activity; significant potency; nociceptive pain upon injection into mice |
[72] |
|
NA |
glatiramer acetate |
in vitro |
NA |
Efficient killing of clinical isolates |
[73] |
|
King cobra |
OH-CATH30 |
in vitro; in vivo |
Mouse model |
Strong inhibition activity; low toxicity, great immunogenicity; |
[74] |
|
NA |
stapled AMP Mag(i+4)1,15(A9 K, B21A, N22 K, S23 K) |
in vitro; in vivo |
Mouse peritonitis sepsis model |
Great bactericidal activity; 88% of mice cured after intraperitoneal injection; |
[75] |
|
Viola odorata |
Cy02 (cyclotide) |
in vitro |
NA |
Strong bactericidal action |
[76] |
|
P. aeruginosa bacteriophage |
artilysin 175 |
in vitro |
NA |
High, rapid and broad antibacterial activity against MRAB |
[77] |
|
Calliphora vicina |
FLIP 7 |
in vitro |
NA |
Antibiofilm activity |
[78] |
|
Camel (colostrum milk) |
lactoperoxidase |
in vitro; in vivo |
Acute pneumonia mouse model |
Major inhibition effects; significant clearance of A. baumannii in lung and blood culture; |
[79] |
|
Rana catesbeiana |
ranalexin |
in vitro |
NA |
Strong antimicrobial activity |
[80] |
|
NA |
PNA (RXR)4 XB |
in vitro; in vivo |
Galleria mellonella sepsis model |
Excellent bactericidal activity in vitro; high dose of PNA conjugate required in sepsis model |
[81] |
|
NA |
protegrin-1 |
in vitro |
NA |
Good activity against MRAB; no antibiofilm activity; |
[82] |
|
NA |
aurein 1.2, CAMEL, citropin 1.1., LL-37, omiganan, r-omiganan, pexiganan and temporin A |
in vitro |
NA |
CAMEL and pexiganan displayed the highest antibacterial activity |
[83] |
NA, not applicable; PRAB, polymyxin-resistant A. baumannii; HBcARD, human hepatitis B virus core protein arginine-rich domain; FLIP 7, fly larvae immune peptides 7; PNA (RXR)4 XB, peptide nucleic acid conjugated with cell-penetrating peptide.
2.3. CRISPR System-a New Approach in the “Post-Antibiotic Era”?
As mentioned previously, the current strategies used to combat MDRAB infections have many limitations. In the recent years, one of the most attractive alternatives to combat bacterial resistance is the use of CRISPR (clustered regularly interspaced short palindromic repeat) system described for the first time in 1987, by Ishino et al [84]. CRISPR/Cas is an immune defense system encountered in bacteria able to recognize and degrade foreign nucleic acids through associated caspases.
One of the most significant advantages of this system is its high specificity, based on the existence of short, repetitive sequences in CRISPR loci separated from each other by single sequences of 26–72 pairs of lengths derived from MGEs such as plasmids or transposons [85]. The defense mechanism against exogenous genetic elements is accomplished in three stages: acquisition, expression and interference [86]. The acquisition stage involves the insertion into repetitive loci of the host chromosome of single sequences (spacers) derived from MGEs, separated by repetitive sequences. The expression consists of transcribing the complex formed of repetitive and spacer sequences into a single RNA transcript that will be further processed by caspases in short CRISPR RNAs. Caspases, including ribonucleases, are a family of protease enzymes, playing essential roles in programmed cell death [87]. In the interference phase, foreign nucleic acids are identified based on complementarity with CRISPR RNAs, and their degradation is accomplished by caspases [88]. Discrimination between self and non-self is accomplished through sequences from the foreign nucleic acid called protospacers. These sequences are placed between some sequence motifs called PAMs (adjacent protospacer motif). Direct target recognition is achieved only by identifying these sequence motifs not stored in CRISPR loci, so there is no risk of degradation of its nucleic acid [89] (Figure 1).
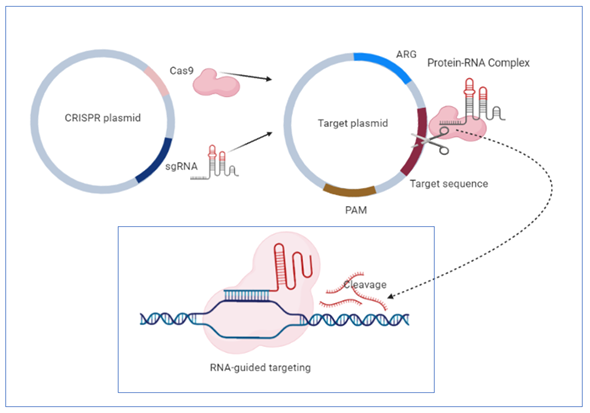
In A. baumannii, two types of CRISPR systems were identified within MGEs containing different spacer sequences. In addition, the distribution of some analyzed isolates in different clusters was observed, suggesting that this system was acquired by HGT throughout evolution [90][91]. Karah et al. analyzed 76 A. baumannii isolates to study the I-Fb subtype of the CRISPR system. Forty types of CRISPR sequences were revealed, two being found mostly in 35.52% of the analyzed isolates, suggesting the existence of two primary clones. The spacer sequences are arranged in chronological order of their inclusion from the invading nucleic acids so that the old ones are positioned at the end. This temporal positioning of the sequences allows the use of the CRISPR system for the micro- and macroepidemiological classification of clinical isolates [92].
These results have opened new perspectives regarding the possibility of using the CRISPR system for subtyping A. baumannii strains. Wang et al. developed a CRISPR platform that allows rapid genomic editing by introducing deletions, insertions, and point mutations to analyze the mechanisms involved in oxidative stress (OxyR) in A. baumannii strains. For the introduction of deletions, the authors constructed a CRISPR plasmid in which they incorporated the CRISPR elements from Streptococcus pyogenes. To repair double-strand breaks, they used RecA recombinase from A. baumannii. The introduced mutations in the oxyR gene and its deletion, lead to a high susceptibility level of A. baumannii strains to oxidative stress, demonstrating the importance of this gene as a central transcriptional regulator of the response to oxidative stress [93]. Mangas et al., conducted an in silico study to analyze the pan-genome of 2500 A. baumannii strains. Depending on the number of shared genes, the authors observed that genomes are divided into two broad groups. The group of strains with a lower number of genes shows the sequences and genes characteristic of the CRISPR system, genes specific to the toxin–antitoxin system and genes involved in the biofilm formation, which is why it is considered that the CRISPR system may have an essential role in virulence. Unlike the strains from group two, positive for a high number of plasmids, the strains from the first group contain mainly genes involved in regulating the elements of the CRISPR system. This finding led to the idea that the CRISPR system may be involved in the restriction of the plasmid entry into bacterial cells [94]. Karlapudi et al. conducted an in silico study to understand the role of the AbaI gene in biofilm formation in A. baumannii. For this, they used a series of genetic editing tools to create AbaI gene knockouts. The analyzed tools (CHOCHOP, CCTop, E-CRISP, CRISPR Direct, Off-Spotter, Crispr-era) can provide information about the target sequences, specific primers, existing mutations, the location of the target sequence in order to perform the knockout, as well as the necessary sgRNA sequences for performing genomic editing experiments [95]. The information obtained from the in silico genomic experiments demonstrates the need to improve genetic editing tools. In addition, further studies should consider the construction of sgRNA with a custom design, depending on the diversity of cell types.
Despite the notable results obtained from using the CRISPR system to combat antibiotic resistance, however, controlling bacterial populations using this strategy has some limitations. First of all, the appearance of mutations outside the target represents a significant limitation of the CRISPR system. In addition to cleaving target sequences, the CRISPR system can act on identical or homologous DNA sequences, leading to mutations in unwanted sites, called off-target mutations. Mutations outside the target can lead to cell death or transformation, which is why it is recommended to select target sites at which as few mutations can occur outside the target [96].
Another challenge in using the CRISPR system in controlling bacterial populations is the need for PAM sequences that are involved in differentiating between self and non-self. There is a limitation of the number of target sites due to the need for these sequences. Because the CRISPR system requires specific PAM sequences to function, their genetic engineering processing can eliminate this limitation [96].
Another major limitation in using the CRISPR system in the control of bacterial populations is represented by the delivery of the protein–RNA complex through the bacterial membrane. There is the problem of delivering the CRISPR system in the case of both Gram-negative and Gram-positive bacteria. It has been observed that the techniques used to encapsulate the gRNA-protein complex have a significant impact on loading and packaging efficiency, thus limiting their practical use [97][98]. Consequently, studies have focused on the use of phages as a vehicle for the delivery of the CRISPR system at the target level. Starting from phages as a delivery vehicle of the CRISPR system in vitro, the problem of controlling bacterial populations using the CRISPR system in vivo was raised. Starting from the fact that oral administration of phages for targeting bacteria in the intestinal tract was used successfully [99], one strategy is to use phages as a vehicle for delivering the CRISPR system in the intestinal microbiota, for eliminating the ARGs. However, it is required to have a collection of phages specially designed to target ARGs, to establish the optimal concentration required and to know the several barriers that occur in vivo, such as inactivation of bacteriophages by gastric acid, neutralization of phages by the spleen and the immune system [100].
3. Discussion
To date, a significant challenge in the clinic is to develop methods to establish the appropriate MIC values needed to increase treatment efficiency. Most laboratories are not able to determine MIC values accurately and reproducibly enough and to eliminate variations. One cause of variations in MIC values is the existence of several strategies and methods to determine these values. Terwee et al. studied the differences obtained in MIC values following the use of several methods that fall into two general categories: "anchor" methods and distribution-based methods. Anchor methods use an external criterion to establish a significant change (patient opinion), and distribution-based methods use statistical data to determine the MIC value. In the case of applying the two strategies, Terwee et al. observed significant differences in MIC values [101]. These significant differences could be closely related to population characteristics such as age, the severity of the condition and treatment and the method used. In this situation, several factors that contribute to variability make it difficult for clinicians to establish a single MIC value or at least a range of values as small as possible [101].The semiautomatic susceptibility detectors often provide truncated MIC values. The same problem exists with gradient tests, such as the E-test, which may omit a certain percentage of resistant strains, leading to treatment failure in the clinic [102]. Even if the susceptibility tests are performed correctly, variations may occur due to discontinuous results reported at a specific interval, usually at a 2-fold scalar dilution. When variations occur, MIC values may exceed these intervals, leading to incorrect doses of antibiotics, which may be harmful to the patient [101]. For this reason, it is imperative to standardize the methodology for identifying MIC values in microbiology laboratories. The standardization process is essential for prescribing a correct treatment, controlling severe infections, and for stopping the expansion of the resistance phenomenon. Moreover, the in vitro susceptibility tests used for prescribing a treatment do not provide information about the bacteriostatic or bactericidal activity of an antibiotic [102].
The heterogeneity and increased efficiency of the resistance mechanisms of A. baumannii strains against almost all existing antibiotics threatens with the transition to the "post-antibiotic era", indicating the acute need to search for new therapeutic approaches. One of the challenges that hinder the success of the treatment of severe infections is the emergence of the phenomenon of heteroresistance. Heteroresistance occurs when subpopulations of isogenic bacteria exhibit lower susceptibility than the general population [103]. In A. baumannii, heteroresistance has been reported in antibiotics such as aminoglycosides, tobramycin, gentamicin and imipenem [104][105], but also in other antimicrobial agents such as AMPs [106]. Currently, the biggest threat is the reporting of colistin heteroresistance, which implies the existence of resistant subpopulations in a susceptible isolate (MIC ≤ 2 mg/L) by in vitro susceptibility tests [107]. In the case of severe nosocomial infections produced by heteroresistant strains in the clinic, colistin treatment may cause resistance expansion and, thus, treatment failure [108]. For detection of the phenomenon of heteroresistance, various methods are used, such as BMD (broth microdilution), E-test or PAP (population analysis profile) [107]. The use of appropriate susceptibility tests to identify heterogeneous subpopulations is essential for the success of clinical treatment. The study by Caglan et al. highlighted differences between the results obtained after the application of BMD and E-test. Using E-test, the resistance to colistin was 4.2%, while by the BMD method, a percentage of 25.8% was obtained, analyzing the same isolates. Therefore, in the case of the E-test, a large part of the resistant strains has not been identified, which is a significant mistake in the clinic [109]. Thus, clinicians should keep in mind that although the use of gradient tests is more comfortable, the results can be confusing and can negatively influence patients' treatment.
Another problem that clinicians need to consider is the emergence of heteroresistance in patients who do not have a history of colistin treatment. However, it is more common in patients who have received treatment [110]. The emergence of heteroresistance to a range of antibiotics, including last-line antibiotics, significantly impedes the management of severe nosocomial infections caused by MDRAB strains and requires increased clinical attention to identify resistant subpopulations.
4. Conclusions
Different MDR microorganisms, among which A. baumannii are opportunistic pathogens, able to compete in new environments where previously only commensals or non-pathogenic microorganisms existed. The survival and persistence in nosocomial environments characterized by high antimicrobial pressure have led to the emergence of A. baumannii as a key pathogen, whereas a few decades ago, it caused practically no disease. The incidence of MDR and virulent clones of A. baumannii is also increasing worldwide, at least in these specific settings.
One of the clinic's difficult challenges is establishing the correct MIC values based on which to prescribe a correct treatment. The existence of numerous factors that influence the MIC values such as the lack of standardization of the methodology makes the success of the therapy difficult. Increased attention should be paid to factors that may influence MIC values such as patient characteristics (age, disease severity) and methods applied. In addition, identifying MIC values and MBC values can provide clinicians with additional information about the antibiotics needed to prescribe the most appropriate treatment.
The emergence of heteroresistance in some bacterial subpopulations is another challenge in the management of infections caused by A. baumannii.
The enormous adaptability of A. baumannii, as well as the very diverse mechanisms for the acquisition and transfer of AR determinants, contribute to the inefficiency of most current therapeutic strategies, determining the transition to the "post-antibiotic era" and highlighting the necessity to develop new therapeutic approaches. The latest strategies include obtaining the use of AMPs, bacteriophage therapy and CRISPR technology. Although experiments have shown the potential of these strategies in combating MDRAB, there are several challenges, such as the narrow spectrum of action, low specificity, high cytotoxicity, sensitivity to enzymatic degradation and bacterial resistance. These limitations must be addressed in future studies to develop efficient strategies for the optimal management of MDRAB infections.
References
- Eze, E.C.; Chenia, H.Y.; El Zowalaty, M.E. Acinetobacter Baumannii Biofilms: Effects of Physicochemical Factors, Virulence, Antibiotic Resistance Determinants, Gene Regulation, and Future Antimicrobial Treatments. Drug Resist. 2018, 11, 2277–2299. DOI:10.2147/IDR.S169894.
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter Baumannii: Emergence of a Successful Pathogen. Microbiol. Rev. 2008, 21 (3), 538–582. DOI:10.1128/CMR.00058-07.
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An Increasing Threat in Hospitals: Multidrug-Resistant Acinetobacter Baumannii. Rev. Microbiol. 2007, 5 (12), 939–951. DOI:10.1038/nrmicro1789.
- Willyard, C. The Drug-Resistant Bacteria That Pose the Greatest Health Threats. Nature. England February 2017, p. 15. DOI:10.1038/nature.2017.21550.
- Barth, V.C.J.; Rodrigues, B.Á.; Bonatto, G.D.; Gallo, S.W.; Pagnussatti, V.E.; Ferreira, C.A.S.; de Oliveira, S.D. Heterogeneous Persister Cells Formation in Acinetobacter Baumannii. PLoS One 2013, 8 (12), e84361. DOI:10.1371/journal.pone.0084361.
- Adams, M.D.; Nickel, G.C.; Bajaksouzian, S.; Lavender, H.; Murthy, A.R.; Jacobs, M.R.; Bonomo, R.A. Resistance to Colistin in Acinetobacter Baumannii Associated with Mutations in the PmrAB Two-Component System. Agents Chemother. 2009, 53 (9), 3628–3634. DOI:10.1128/AAC.00284-09.
- Vrancianu, C.O.; Popa, L.I.; Bleotu, C.; Chifiriuc, M.C. Targeting Plasmids to Limit Acquisition and Transmission of Antimicrobial Resistance. Microbiol. 2020, 11, 761. DOI:10.3389/fmicb.2020.00761.
- Harada, L.K.; Silva, E.C.; Campos, W.F.; Del Fiol, F.S.; Vila, M.; Dąbrowska, K.; Krylov, V.N.; Balcão, V.M. Biotechnological Applications of Bacteriophages: State of the Art. Res. 2018, 212–213, 38–58. DOI:10.1016/j.micres.2018.04.007.
- Buckling, A.; Rainey, P.B. Antagonistic Coevolution between a Bacterium and a Bacteriophage. Biol. Sci. 2002, 269 (1494), 931–936. DOI:10.1098/rspb.2001.1945.
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage Therapy: An Alternative to Antibiotics in the Age of Multi-Drug Resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8 (3), 162–173. DOI:10.4292/wjgpt.v8.i3.162.
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G.J. Bacteriophage Therapy. Agents Chemother. 2001, 45 (3), 649–659. DOI:10.1128/AAC.45.3.649-659.2001.
- Clark, J.R.; March, J.B. Bacteriophages and Biotechnology: Vaccines, Gene Therapy and Antibacterials. Trends Biotechnol. 2006, 24 (5), 212–218. DOI:10.1016/j.tibtech.2006.03.003.
- Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóż, P.; Kłak, M.; Wojtasik, E.; et al. Clinical Aspects of Phage Therapy. Virus Res. 2012, 83, 73–121. DOI:10.1016/B978-0-12-394438-2.00003-7.
- Shen, G.-H.; Wang, J.-L.; Wen, F.-S.; Chang, K.-M.; Kuo, C.-F.; Lin, C.-H.; Luo, H.-R.; Hung, C.-H. Isolation and Characterization of Φkm18p, a Novel Lytic Phage with Therapeutic Potential against Extensively Drug Resistant Acinetobacter Baumannii. PLoS One 2012, 7 (10), e46537. DOI:10.1371/journal.pone.0046537.
- Merabishvili, M.; Vandenheuvel, D.; Kropinski, A.M.; Mast, J.; De Vos, D.; Verbeken, G.; Noben, J.-P.; Lavigne, R.; Vaneechoutte, M.; Pirnay, J.-P. Characterization of Newly Isolated Lytic Bacteriophages Active against Acinetobacter PLoS One 2014, 9 (8), e104853. DOI:10.1371/journal.pone.0104853.
- Ghajavand, H.; Esfahani, B.N.; Havaei, A.; Fazeli, H.; Jafari, R.; Moghim, S. Isolation of Bacteriophages against Multidrug Resistant Acinetobacter Baumannii. Pharm. Sci. 2017, 12 (5), 373–380. DOI:10.4103/1735-5362.213982.
- Turner, D.; Wand, M.E.; Briers, Y.; Lavigne, R.; Sutton, J.M.; Reynolds, D.M. Characterisation and Genome Sequence of the Lytic Acinetobacter Baumannii Bacteriophage VB_AbaS_Loki. PLoS One 2017, 12 (2), e0172303. DOI:10.1371/journal.pone.0172303.
- Hernandez-Morales, A.C.; Lessor, L.L.; Wood, T.L.; Migl, D.; Mijalis, E.M.; Cahill, J.; Russell, W.K.; Young, R.F.; Gill, J.J. Genomic and Biochemical Characterization of Acinetobacter Podophage Petty Reveals a Novel Lysis Mechanism and Tail-Associated Depolymerase Activity. Virol. 2018, 92 (6). DOI:10.1128/JVI.01064-17.
- Hua, Y.; Luo, T.; Yang, Y.; Dong, D.; Wang, R.; Wang, Y.; Xu, M.; Guo, X.; Hu, F.; He, P. Phage Therapy as a Promising New Treatment for Lung Infection Caused by Carbapenem-Resistant Acinetobacter Baumannii in Mice. Microbiol. 2017, 8, 2659. DOI:10.3389/fmicb.2017.02659.
- Kusradze, I.; Karumidze, N.; Rigvava, S.; Dvalidze, T.; Katsitadze, M.; Amiranashvili, I.; Goderdzishvili, M. Characterization and Testing the Efficiency of Acinetobacter Baumannii Phage VB-GEC_Ab-M-G7 as an Antibacterial Agent. Microbiol. 2016, 7, 1590. DOI:10.3389/fmicb.2016.01590.
- Yin, S.; Huang, G.; Zhang, Y.; Jiang, B.; Yang, Z.; Dong, Z.; You, B.; Yuan, Z.; Hu, F.; Zhao, Y.; et al. Phage Abp1 Rescues Human Cells and Mice from Infection by Pan-Drug Resistant Acinetobacter Baumannii. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44 (6), 2337–2345. DOI:10.1159/000486117.
- Cha, K.; Oh, H.K.; Jang, J.Y.; Jo, Y.; Kim, W.K.; Ha, G.U.; Ko, K.S.; Myung, H. Characterization of Two Novel Bacteriophages Infecting Multidrug-Resistant (MDR) Acinetobacter Baumannii and Evaluation of Their Therapeutic Efficacy in Vivo. Microbiol. 2018, 9, 696. DOI:10.3389/fmicb.2018.00696.
- Zhou, W.; Feng, Y.; Zong, Z. Two New Lytic Bacteriophages of the Myoviridae Family Against Carbapenem-Resistant Acinetobacter Baumannii. Microbiol. 2018, 9, 850. DOI:10.3389/fmicb.2018.00850.
- Wu, M.; Hu, K.; Xie, Y.; Liu, Y.; Mu, D.; Guo, H.; Zhang, Z.; Zhang, Y.; Chang, D.; Shi, Y. A Novel Phage PD-6A3, and Its Endolysin Ply6A3, With Extended Lytic Activity Against Acinetobacter Baumannii. Microbiol. 2018, 9, 3302. DOI:10.3389/fmicb.2018.03302.
- Jeon, J.; Park, J.-H.; Yong, D. Efficacy of Bacteriophage Treatment against Carbapenem-Resistant Acinetobacter Baumannii in Galleria Mellonella Larvae and a Mouse Model of Acute Pneumonia. BMC Microbiol. 2019, 19 (1), 70. DOI:10.1186/s12866-019-1443-5.
- Jasim, H.N.; Hafidh, R.R.; Abdulamir, A.S. Formation of Therapeutic Phage Cocktail and Endolysin to Highly Multi-Drug Resistant Acinetobacter Baumannii: In Vitro and in Vivo Study. J. Basic Med. Sci. 2018, 21 (11), 1100–1108. DOI:10.22038/IJBMS.2018.27307.6665.
- Summers, WC. Bacteriophage:Early Research. In Bacteriophage: Biology and Applications; Boca Raton, FL : CRC Press, 2005., 2005; p. 510.
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage Treatment of Human Infections. Bacteriophage 2011, 1 (2), 66–85. DOI:10.4161/bact.1.2.15845.
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. P T 2015, 40 (4), 277–283.
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a New Antibiotic Resistance Mechanism in India, Pakistan, and the UK: A Molecular, Biological, and Epidemiological Study. Infect. Dis. 2010, 10 (9), 597–602. DOI:10.1016/S1473-3099(10)70143-2.
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter Baumannii Infection. Agents Chemother. 2017, 61 (10). DOI:10.1128/AAC.00954-17.
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh. EBioMedicine 2016, 4, 124–137. DOI:10.1016/j.ebiom.2015.12.023.
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and Tolerability of a Cocktail of Bacteriophages to Treat Burn Wounds Infected by Pseudomonas Aeruginosa (PhagoBurn): A Randomised, Controlled, Double-Blind Phase 1/2 Trial. Infect. Dis. 2019, 19 (1), 35–45. DOI:10.1016/S1473-3099(18)30482-1.
- Azam, A.H.; Tanji, Y. Bacteriophage-Host Arm Race: An Update on the Mechanism of Phage Resistance in Bacteria and Revenge of the Phage with the Perspective for Phage Therapy. Microbiol. Biotechnol. 2019, 103 (5), 2121–2131. DOI:10.1007/s00253-019-09629-x.
- Taylor, V.L.; Fitzpatrick, A.D.; Islam, Z.; Maxwell, K.L. The Diverse Impacts of Phage Morons on Bacterial Fitness and Virulence. Virus Res. 2019, 103, 1–31. DOI:10.1016/bs.aivir.2018.08.001.
- Yuan, Y.; Wang, L.; Li, X.; Tan, D.; Cong, C.; Xu, Y. Efficacy of a Phage Cocktail in Controlling Phage Resistance Development in Multidrug Resistant Acinetobacter Baumannii. Virus Res. 2019, 272, 197734. DOI:10.1016/j.virusres.2019.197734.
- Chadha, P.; Katare, O.P.; Chhibber, S. In Vivo Efficacy of Single Phage versus Phage Cocktail in Resolving Burn Wound Infection in BALB/c Mice. Pathog. 2016, 99, 68–77. DOI:10.1016/j.micpath.2016.08.001.
- Sneppen, K.; Semsey, S.; Seshasayee, A.S.N.; Krishna, S. Restriction Modification Systems as Engines of Diversity. Microbiol. 2015, 6, 528. DOI:10.3389/fmicb.2015.00528.
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11 (1). DOI:10.3390/v11010010.
- Kumaran, D.; Taha, M.; Yi, Q.; Ramirez-Arcos, S.; Diallo, J.-S.; Carli, A.; Abdelbary, H. Does Treatment Order Matter? Investigating the Ability of Bacteriophage to Augment Antibiotic Activity against Staphylococcus Aureus Biofilms. Microbiol. 2018, 9, 127. DOI:10.3389/fmicb.2018.00127.
- Mansour, S.C.; Pena, O.M.; Hancock, R.E.W. Host Defense Peptides: Front-Line Immunomodulators. Trends Immunol. 2014, 35 (9), 443–450. DOI:10.1016/j.it.2014.07.004.
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.R.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine Antimicrobial Peptides: Nature Provides Templates for the Design of Novel Compounds against Pathogenic Bacteria. J. Mol. Sci. 2016, 17 (5). DOI:10.3390/ijms17050785.
- Fan, L.; Sun, J.; Zhou, M.; Zhou, J.; Lao, X.; Zheng, H.; Xu, H. DRAMP: A Comprehensive Data Repository of Antimicrobial Peptides. Rep. 2016, 6, 24482. DOI:10.1038/srep24482.
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8 (1). DOI:10.3390/biom8010004.
- Govender, T.; Dawood, A.; Esterhuyse, A.J.; Katerere, D.R. Antimicrobial Properties of the Skin Secretions of Frogs. Afr. J. Sci. 2012, 108, 25–30.
- Dathe, M.; Wieprecht, T. Structural Features of Helical Antimicrobial Peptides: Their Potential to Modulate Activity on Model Membranes and Biological Cells. Biophys. Acta 1999, 1462 (1–2), 71–87. DOI:10.1016/s0005-2736(99)00201-1.
- Starr, C.G.; Wimley, W.C. Antimicrobial Peptides Are Degraded by the Cytosolic Proteases of Human Erythrocytes. Biophys. acta. Biomembr. 2017, 1859 (12), 2319–2326. DOI:10.1016/j.bbamem.2017.09.008.
- McPhee, J.B.; Hancock, R.E.W. Function and Therapeutic Potential of Host Defence Peptides. Pept. Sci. 2005, 11 (11), 677–687. DOI:10.1002/psc.704.
- Cantisani, M.; Finamore, E.; Mignogna, E.; Falanga, A.; Nicoletti, G.F.; Pedone, C.; Morelli, G.; Leone, M.; Galdiero, M.; Galdiero, S. Structural Insights into and Activity Analysis of the Antimicrobial Peptide Antimicrob. Agents Chemother. 2014, 58 (9), 5280–5290. DOI:10.1128/AAC.02395-14.
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. De Novo Generation of Short Antimicrobial Peptides with Enhanced Stability and Cell J. Antimicrob. Chemother. 2014, 69 (1), 121–132. DOI:10.1093/jac/dkt322.
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The Antimicrobial Peptide ZY4 Combats Multidrug-Resistant Pseudomonas Aeruginosa and Acinetobacter Baumannii Infection. Natl. Acad. Sci. U. S. A. 2019, 116 (52), 26516–26522. DOI:10.1073/pnas.1909585117.
- Khan, A.; Xu, M.; Wang, T.; You, C.; Wang, X.; Ren, H.; Zhou, H.; Khan, A.; Han, C.; Li, P. Catechol Cross-Linked Antimicrobial Peptide Hydrogels Prevent Multidrug-Resistant Acinetobacter Baumannii Infection in Burn Wounds. Rep. 2019, 39 (6). DOI:10.1042/BSR20190504.
- Lin, C.-H.; Lee, M.-C.; Tzen, J.T.C.; Lee, H.-M.; Chang, S.-M.; Tu, W.-C.; Lin, C.-F. Efficacy of Mastoparan-AF Alone and in Combination with Clinically Used Antibiotics on Nosocomial Multidrug-Resistant Acinetobacter Baumannii. Saudi J. Biol. Sci. 2017, 24 (5), 1023–1029. DOI:10.1016/j.sjbs.2016.12.013.
- Ostorhazi, E.; Hoffmann, R.; Herth, N.; Wade, J.D.; Kraus, C.N.; Otvos, L.J. Advantage of a Narrow Spectrum Host Defense (Antimicrobial) Peptide Over a Broad Spectrum Analog in Preclinical Drug Development. Chem. 2018, 6, 359. DOI:10.3389/fchem.2018.00359.
- Mant, C.T.; Jiang, Z.; Gera, L.; Davis, T.; Nelson, K.L.; Bevers, S.; Hodges, R.S. De Novo Designed Amphipathic α-Helical Antimicrobial Peptides Incorporating Dab and Dap Residues on the Polar Face To Treat the Gram-Negative Pathogen, Acinetobacter Baumannii. Med. Chem. 2019, 62 (7), 3354–3366. DOI:10.1021/acs.jmedchem.8b01785.
- Tejman-Yarden, N.; Robinson, A.; Davidov, Y.; Shulman, A.; Varvak, A.; Reyes, F.; Rahav, G.; Nissan, I. Delftibactin-A, a Non-Ribosomal Peptide With Broad Antimicrobial Activity. Microbiol. 2019, 10, 2377. DOI:10.3389/fmicb.2019.02377.
- Shamova, O. V; Orlov, D.S.; Zharkova, M.S.; Balandin, S. V; Yamschikova, E. V; Knappe, D.; Hoffmann, R.; Kokryakov, V.N.; Ovchinnikova, T. V. Minibactenecins ChBac7.Nα and ChBac7. Nβ - Antimicrobial Peptides from Leukocytes of the Goat Capra Hircus. Acta Naturae 2016, 8 (3), 136–146.
- Taheri, B.; Mohammadi, M.; Momenzadeh, N.; Farshadzadeh, Z.; Roozbehani, M.; Dehghani, P.; Hajian, S.; Darvishi, S.; Shamseddin, J. Substitution of Lysine for Isoleucine at the Center of the Nonpolar Face of the Antimicrobial Peptide, Piscidin-1, Leads to an Increase in the Rapidity of Bactericidal Activity and a Reduction in Toxicity. Drug Resist. 2019, 12, 1629–1647. DOI:10.2147/IDR.S195872.
- Peng, J.; Long, H.; Liu, W.; Wu, Z.; Wang, T.; Zeng, Z.; Guo, G.; Wu, J. Antibacterial Mechanism of Peptide Cec4 against Acinetobacter Baumannii. Drug Resist. 2019, 12, 2417–2428. DOI:10.2147/IDR.S214057.
- Nagarajan, D.; Roy, N.; Kulkarni, O.; Nanajkar, N.; Datey, A.; Ravichandran, S.; Thakur, C.; T, S.; Aprameya, I. V; Sarma, S.P.; et al. Ω76: A Designed Antimicrobial Peptide to Combat Carbapenem- and Tigecycline-Resistant Acinetobacter Baumannii. Adv. 2019, 5 (7), eaax1946. DOI:10.1126/sciadv.aax1946.
- Hacioglu, M.; Oyardi, O.; Bozkurt-Guzel, C.; Savage, P.B. Antibiofilm Activities of Ceragenins and Antimicrobial Peptides against Fungal-Bacterial Mono and Multispecies Biofilms. Antibiot. (Tokyo). 2020. DOI:10.1038/s41429-020-0299-0.
- Jenei, S.; Tiricz, H.; Szolomájer, J.; Tímár, E.; Klement, É.; Al Bouni, M.A.; Lima, R.M.; Kata, D.; Harmati, M.; Buzás, K.; et al. Potent Chimeric Antimicrobial Derivatives of the Medicago Truncatula NCR247 Symbiotic Peptide. Microbiol. 2020, 11, 270. DOI:10.3389/fmicb.2020.00270.
- Heulot, M.; Jacquier, N.; Aeby, S.; Le Roy, D.; Roger, T.; Trofimenko, E.; Barras, D.; Greub, G.; Widmann, C. The Anticancer Peptide TAT-RasGAP(317-326) Exerts Broad Antimicrobial Activity. Microbiol. 2017, 8, 994. DOI:10.3389/fmicb.2017.00994.
- Swedan, S.; Shubair, Z.; Almaaytah, A. Synergism of Cationic Antimicrobial Peptide WLBU2 with Antibacterial Agents against Biofilms of Multi-Drug Resistant Acinetobacter Baumannii and Klebsiella Pneumoniae. Drug Resist. 2019, 12, 2019–2030. DOI:10.2147/IDR.S215084.
- Han, H.M.; Ko, S.; Cheong, M.-J.; Bang, J.K.; Seo, C.H.; Luchian, T.; Park, Y. Myxinidin2 and Myxinidin3 Suppress Inflammatory Responses through STAT3 and MAPKs to Promote Wound Healing. Oncotarget 2017, 8 (50), 87582–87597. DOI:10.18632/oncotarget.20908.
- Chen, H.-L.; Su, P.-Y.; Kuo, S.-C.; Lauderdale, T.-L.Y.; Shih, C. Adding a C-Terminal Cysteine (CTC) Can Enhance the Bactericidal Activity of Three Different Antimicrobial Peptides. Microbiol. 2018, 9, 1440. DOI:10.3389/fmicb.2018.01440.
- Mohan, N.M.; Zorgani, A.; Jalowicki, G.; Kerr, A.; Khaldi, N.; Martins, M. Unlocking NuriPep 1653 From Common Pea Protein: A Potent Antimicrobial Peptide to Tackle a Pan-Drug Resistant Acinetobacter Baumannii. Microbiol. 2019, 10, 2086. DOI:10.3389/fmicb.2019.02086.
- Kaushal, A.; Gupta, K.; van Hoek, M.L. Characterization of Cimex Lectularius (Bedbug) Defensin Peptide and Its Antimicrobial Activity against Human Skin Microflora. Biophys. Res. Commun. 2016, 470 (4), 955–960. DOI:10.1016/j.bbrc.2016.01.100.
- Tajbakhsh, M.; Akhavan, M.M.; Fallah, F.; Karimi, A. A Recombinant Snake Cathelicidin Derivative Peptide: Antibiofilm Properties and Expression in Escherichia Coli. Biomolecules 2018, 8 (4). DOI:10.3390/biom8040118.
- Hirsch, R.; Wiesner, J.; Marker, A.; Pfeifer, Y.; Bauer, A.; Hammann, P.E.; Vilcinskas, A. Profiling Antimicrobial Peptides from the Medical Maggot Lucilia Sericata as Potential Antibiotics for MDR Gram-Negative Bacteria. Antimicrob. Chemother. 2019, 74 (1), 96–107.
- Al-Asmari, A.K.; Alamri, M.A.; Almasoudi, A.S.; Abbasmanthiri, R.; Mahfoud, M. Evaluation of the in Vitro Antimicrobial Activity of Selected Saudi Scorpion Venoms Tested against Multidrug-Resistant Micro-Organisms. Glob. Antimicrob. Resist. 2017, 10, 14–18. DOI:10.1016/j.jgar.2017.03.008.
- Dekan, Z.; Headey, S.J.; Scanlon, M.; Baldo, B.A.; Lee, T.-H.; Aguilar, M.-I.; Deuis, J.R.; Vetter, I.; Elliott, A.G.; Amado, M.; et al. Δ-Myrtoxin-Mp1a Is a Helical Heterodimer from the Venom of the Jack Jumper Ant That Has Antimicrobial, Membrane-Disrupting, and Nociceptive Activities.
- Christiansen, S.H.; Murphy, R.A.; Juul-Madsen, K.; Fredborg, M.; Hvam, M.L.; Axelgaard, E.; Skovdal, S.M.; Meyer, R.L.; Sørensen, U.B.S.; Möller, A.; et al. The Immunomodulatory Drug Glatiramer Acetate Is Also an Effective Antimicrobial Agent That Kills Gram-Negative Bacteria. Rep. 2017, 7 (1), 15653. DOI:10.1038/s41598-017-15969-3.
- Zhao, F.; Lan, X.-Q.; Du, Y.; Chen, P.-Y.; Zhao, J.; Zhao, F.; Lee, W.-H.; Zhang, Y. King Cobra Peptide OH-CATH30 as a Potential Candidate Drug through Clinic Drug-Resistant Isolates. Res. 2018, 39 (2), 87–96. DOI:10.24272/j.issn.2095-8137.2018.025.
- Mourtada, R.; Herce, H.D.; Yin, D.J.; Moroco, J.A.; Wales, T.E.; Engen, J.R.; Walensky, L.D. Design of Stapled Antimicrobial Peptides That Are Stable, Nontoxic and Kill Antibiotic-Resistant Bacteria in Mice. Biotechnol. 2019, 37 (10), 1186–1197. DOI:10.1038/s41587-019-0222-z.
- Kirkpatrick, C.L.; Broberg, C.A.; McCool, E.N.; Lee, W.J.; Chao, A.; McConnell, E.W.; Pritchard, D.A.; Hebert, M.; Fleeman, R.; Adams, J.; et al. The “PepSAVI-MS” Pipeline for Natural Product Bioactive Peptide Discovery. Chem. 2017, 89 (2), 1194–1201. DOI:10.1021/acs.analchem.6b03625.
- Defraine, V.; Schuermans, J.; Grymonprez, B.; Govers, S.K.; Aertsen, A.; Fauvart, M.; Michiels, J.; Lavigne, R.; Briers, Y. Efficacy of Artilysin Art-175 against Resistant and Persistent Acinetobacter Antimicrob. Agents Chemother. 2016, 60 (6), 3480–3488. DOI:10.1128/AAC.00285-16.
- Gordya, N.; Yakovlev, A.; Kruglikova, A.; Tulin, D.; Potolitsina, E.; Suborova, T.; Bordo, D.; Rosano, C.; Chernysh, S. Natural Antimicrobial Peptide Complexes in the Fighting of Antibiotic Resistant Biofilms: Calliphora Vicina Medicinal Maggots. PLoS One 2017, 12 (3), e0173559. DOI:10.1371/journal.pone.0173559.
- Mahdi, L.; Mahdi, N.; Al-Kakei, S.; Musafer, H.; Al-Joofy, I.; Essa, R.; Zwain, L.; Salman, I.; Mater, H.; Al-Alak, S.; et al. Treatment Strategy by Lactoperoxidase and Lactoferrin Combination: Immunomodulatory and Antibacterial Activity against Multidrug-Resistant Acinetobacter Baumannii. Pathog. 2018, 114, 147–152. DOI:10.1016/j.micpath.2017.10.056.
- Domhan, C.; Uhl, P.; Kleist, C.; Zimmermann, S.; Umstätter, F.; Leotta, K.; Mier, W.; Wink, M. Replacement of L-Amino Acids by d-Amino Acids in the Antimicrobial Peptide Ranalexin and Its Consequences for Antimicrobial Activity and Biodistribution. Molecules 2019, 24 (16). DOI:10.3390/molecules24162987.
- Rose, M.; Lapuebla, A.; Landman, D.; Quale, J. In Vitro and In Vivo Activity of a Novel Antisense Peptide Nucleic Acid Compound Against Multidrug-Resistant Acinetobacter Baumannii. Drug Resist. 2019, 25 (7), 961–965. DOI:10.1089/mdr.2018.0179.
- Morroni, G.; Simonetti, O.; Brenciani, A.; Brescini, L.; Kamysz, W.; Kamysz, E.; Neubauer, D.; Caffarini, M.; Orciani, M.; Giovanetti, E.; et al. In Vitro Activity of Protegrin-1, Alone and in Combination with Clinically Useful Antibiotics, against Acinetobacter Baumannii Strains Isolated from Surgical Wounds. Microbiol. Immunol. 2019, 208 (6), 877–883. DOI:10.1007/s00430-019-00624-7.
- Jaśkiewicz, M.; Neubauer, D.; Kazor, K.; Bartoszewska, S.; Kamysz, W. Antimicrobial Activity of Selected Antimicrobial Peptides Against Planktonic Culture and Biofilm of Acinetobacter Baumannii. Probiotics Antimicrob. Proteins 2019, 11 (1), 317–324. DOI:10.1007/s12602-018-9444-5.
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide Sequence of the Iap Gene, Responsible for Alkaline Phosphatase Isozyme Conversion in Escherichia Coli, and Identification of the Gene Product. Bacteriol. 1987, 169 (12), 5429–5433. DOI:10.1128/jb.169.12.5429-5433.1987.
- Li, H.-Y.; Kao, C.-Y.; Lin, W.-H.; Zheng, P.-X.; Yan, J.-J.; Wang, M.-C.; Teng, C.-H.; Tseng, C.-C.; Wu, J.-J. Characterization of CRISPR-Cas Systems in Clinical Klebsiella Pneumoniae Isolates Uncovers Its Potential Association With Antibiotic Susceptibility. Microbiol. 2018, 9, 1595. DOI:10.3389/fmicb.2018.01595.
- Crawley, A.B.; Henriksen, E.D.; Stout, E.; Brandt, K.; Barrangou, R. Characterizing the Activity of Abundant, Diverse and Active CRISPR-Cas Systems in Sci. Rep. 2018, 8 (1), 11544. DOI:10.1038/s41598-018-29746-3.
- Nakagawa, A.; Shi, Y.; Kage-Nakadai, E.; Mitani, S.; Xue, D. Caspase-Dependent Conversion of Dicer Ribonuclease into a Death-Promoting Science 2010, 328 (5976), 327–334. DOI:10.1126/science.1182374.
- Walker, F.C.; Hatoum-Aslan, A. Conjugation Assay for Testing CRISPR-Cas Anti-Plasmid Immunity in Staphylococci. Bio-protocol 2017, 7 (9). DOI:10.21769/BioProtoc.2293.
- Marraffini, L.A.; Sontheimer, E.J. CRISPR Interference Limits Horizontal Gene Transfer in Staphylococci by Targeting Science 2008, 322 (5909), 1843–1845. DOI:10.1126/science.1165771.
- Di Nocera, P.P.; Rocco, F.; Giannouli, M.; Triassi, M.; Zarrilli, R. Genome Organization of Epidemic Acinetobacter Baumannii Strains. BMC Microbiol. 2011, 11, 224. DOI:10.1186/1471-2180-11-224.
- Hauck, Y.; Soler, C.; Jault, P.; Mérens, A.; Gérome, P.; Nab, C. Mac; Trueba, F.; Bargues, L.; Thien, H.V.; Vergnaud, G.; et al. Diversity of Acinetobacter Baumannii in Four French Military Hospitals, as Assessed by Multiple Locus Variable Number of Tandem Repeats Analysis. PLoS One 2012, 7 (9), e44597. DOI:10.1371/journal.pone.0044597.
- Choi, K.R.; Lee, S.Y. CRISPR Technologies for Bacterial Systems: Current Achievements and Future Biotechnol. Adv. 2016, 34 (7), 1180–1209. DOI:10.1016/j.biotechadv.2016.08.002.
- Wang, Y.; Wang, Z.; Chen, Y.; Hua, X.; Yu, Y.; Ji, Q. A Highly Efficient CRISPR-Cas9-Based Genome Engineering Platform in Acinetobacter Baumannii to Understand the H(2)O(2)-Sensing Mechanism of OxyR. Cell Chem. Biol. 2019, 26 (12), 1732-1742.e5. DOI:10.1016/j.chembiol.2019.09.003.
- Mangas, E.L.; Rubio, A.; Álvarez-Marín, R.; Labrador-Herrera, G.; Pachón, J.; Pachón-Ibáñez, M.E.; Divina, F.; Pérez-Pulido, A.J. Pangenome of Acinetobacter Baumannii Uncovers Two Groups of Genomes, One of Them with Genes Involved in CRISPR/Cas Defence Systems Associated with the Absence of Plasmids and Exclusive Genes for Biofilm Formation. genomics 2019, 5 (11). DOI:10.1099/mgen.0.000309.
- Karlapudi, A.P.; T C, V.; Tammineedi, J.; Srirama, K.; Kanumuri, L.; Prabhakar Kodali, V. In Silico SgRNA Tool Design for CRISPR Control of Quorum Sensing in Acinetobacter Genes Dis. 2018, 5 (2), 123–129. DOI:10.1016/j.gendis.2018.03.004.
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for Genome Editing: Progress, Implications and Challenges. Mol. Genet. 2014, 23 (R1), R40-6. DOI:10.1093/hmg/ddu125.
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. J. Nanomedicine 2015, 10, 975–999. DOI:10.2147/IJN.S68861.
- Taranejoo, S.; Liu, J.; Verma, P.; Hourigan, K. A Review of the Developments of Characteristics of PEI Derivatives for Gene Delivery Applications. Appl. Polym. Sci. 2015, 132 (25). DOI:10.1002/app.42096.
- So, Y.; Park, S.-Y.; Park, E.-H.; Park, S.-H.; Kim, E.-J.; Pan, J.-G.; Choi, S.-K. A Highly Efficient CRISPR-Cas9-Mediated Large Genomic Deletion in Bacillus Subtilis. Microbiol. 2017, 8, 1167. DOI:10.3389/fmicb.2017.01167.
- Dong, H.; Xiang, H.; Mu, D.; Wang, D.; Wang, T. Exploiting a Conjugative CRISPR/Cas9 System to Eliminate Plasmid Harbouring the Mcr-1 Gene from Escherichia Coli. J. Antimicrob. Agents 2019, 53 (1), 1–8. DOI:10.1016/j.ijantimicag.2018.09.017.
- Terwee, C.B.; Roorda, L.D.; Dekker, J.; Bierma-Zeinstra, S.M.; Peat, G.; Jordan, K.P.; Croft, P.; de Vet, H.C.W. Mind the MIC: Large Variation among Populations and Methods. Clin. Epidemiol. 2010, 63 (5), 524–534. DOI:10.1016/j.jclinepi.2009.08.010.
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Protoc. 2008, 3 (2), 163–175. DOI:10.1038/nprot.2007.521.
- El-Halfawy, O.M.; Valvano, M.A. Antimicrobial Heteroresistance: An Emerging Field in Need of Clarity. Microbiol. Rev. 2015, 28 (1), 191–207. DOI:10.1128/CMR.00058-14.
- Anderson, S.E.; Sherman, E.X.; Weiss, D.S.; Rather, P.N. Aminoglycoside Heteroresistance in Acinetobacter Baumannii AB5075. mSphere 2018, 3 (4). DOI:10.1128/mSphere.00271-18.
- Lee, H.-Y.; Chen, C.-L.; Wang, S.-B.; Su, L.-H.; Chen, S.-H.; Liu, S.-Y.; Wu, T.-L.; Lin, T.-Y.; Chiu, C.-H. Imipenem Heteroresistance Induced by Imipenem in Multidrug-Resistant Acinetobacter Baumannii: Mechanism and Clinical Implications. J. Antimicrob. Agents 2011, 37 (4), 302–308. DOI:10.1016/j.ijantimicag.2010.12.015.
- Hung, K.-H.; Wang, M.-C.; Huang, A.-H.; Yan, J.-J.; Wu, J.-J. Heteroresistance to Cephalosporins and Penicillins in Acinetobacter Baumannii. Clin. Microbiol. 2012, 50 (3), 721–726. DOI:10.1128/JCM.05085-11.
- Li, J.; Rayner, C.R.; Nation, R.L.; Owen, R.J.; Spelman, D.; Tan, K.E.; Liolios, L. Heteroresistance to Colistin in Multidrug-Resistant Acinetobacter Baumannii. Agents Chemother. 2006, 50 (9), 2946–2950. DOI:10.1128/AAC.00103-06.
- Sola, C.; Lamberghini, R.O.; Ciarlantini, M.; Egea, A.L.; Gonzalez, P.; Diaz, E.G.; Huerta, V.; Gonzalez, J.; Corso, A.; Vilaro, M.; et al. Heterogeneous Vancomycin-Intermediate Susceptibility in a Community-Associated Methicillin-Resistant Staphylococcus Aureus Epidemic Clone, in a Case of Infective Endocarditis in Argentina. Clin. Microbiol. Antimicrob. 2011, 10, 15. DOI:10.1186/1476-0711-10-15.
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-Based Dose Adjustment: Facts and Fables. Antimicrob. Chemother. 2018, 73 (3), 564–568. DOI:10.1093/jac/dkx427.
- Hawley, J.S.; Murray, C.K.; Jorgensen, J.H. Colistin Heteroresistance in Acinetobacter and Its Association with Previous Colistin Therapy. Agents Chemother. 2008, 52 (1), 351–352. DOI:10.1128/AAC.00766-07.




