Gene 33 (also named Mig6, RALT, and ERRFI1) is an adapter/scaffold protein with a calculated molecular weight of about 50 kD. It contains multiple domains known to mediate protein–protein interaction, suggesting that it has the potential to interact with many cellular partners and have multiple cellular functions. The research over the last two decades has confirmed that it indeed regulates multiple cell signaling pathways and is involved in many pathophysiological processes. Gene 33 has long been viewed as an exclusively cytosolic protein. However, recent evidence suggests that it also has nuclear and chromatin-associated functions. These new findings highlight a significantly broader functional spectrum of this protein.
1. Introduction
Gene 33 (also named Mig6, RALT, and ERRFI1) was discovered as a glucocorticoid-induced transcript from the rat liver using the differential hybridization technique in 1985
[1]. The gene that encodes this transcript was initially named
p33 and was later renamed
gene 33 to avoid confusion with its protein product
[2]. The human homologue of
gene 33 was later identified from quiescent fibroblasts treated with serum and named as mitogen-inducible gene 6 (
Mig6 or
Mig-6), for its high inducibility by serum
[3]. Subsequent studies revealed that the transcript of
gene 33 can be induced by a wide variety of extracellular stimuli and is widely expressed
[2][3][4][5][6][7][8][9][10][11][12]. The induction of
gene 33 by multiple signaling inputs is consistent with the fact that the promotor region of
gene 33 contains an array of regulatory elements
[2][13].
gene 33 is considered an immediate early response gene, defined as quick induction in response to stimuli without the requirement of de novo protein synthesis
[3][14]. Gene 33 appeared rather late in the evolution, existing only in vertebrates. It shares considerable homology to the C-terminal portion of activated CDC42-associated kinase 1 (ACK1). Structurally, it is more or less an ACK1 without the kinase domain and the SRC-homology 3 domain (SH3) at the
N-terminus (
Figure 1). It is likely that Gene 33 was descended from ACK1 during evolution to fulfill the functional needs of more advanced animals.
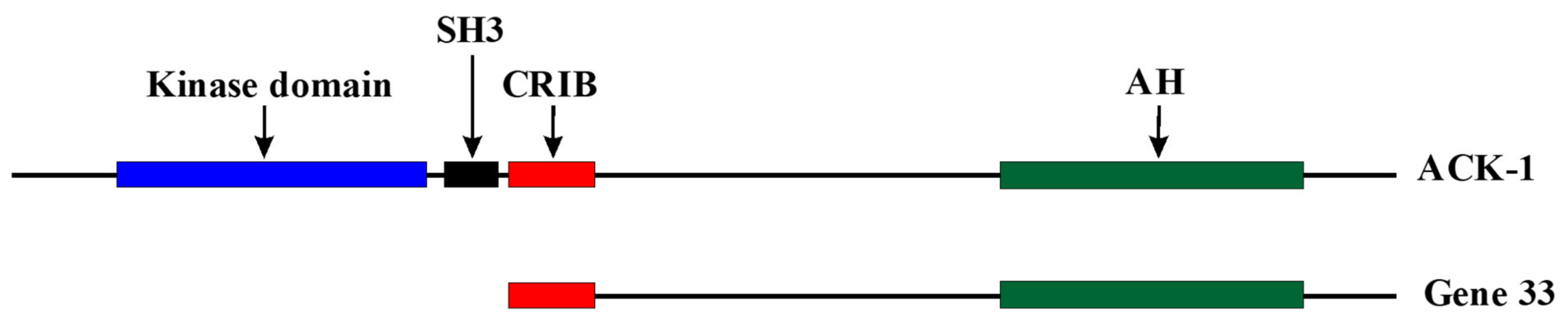 Figure 1.
Figure 1. Comparison of linear structures of Gene 33 and ACK-1. SH3: Src homology 3 domain, CRIB: Cdc42/Rac interactive and binding domain. AH: Ack homology domain.
Although the highly inducible nature of Gene 33 by multiple stimuli, particularly insulin, drew considerable interest in this gene soon after its discovery, the function of its protein product remained elusive until early 2000, when three studies were published on the function of this protein
[4][15][16]. The laboratory of John Kyriakis showed that Gene 33 can be induced in the kidney after unilateral nephrectomy and/or streptozotocin-induced diabetes
[4]. Gene 33 can also be induced by a mechanical strain in a JNK-dependent fashion in rat renal mesangial cells
[4]. The study also described the domain structure of the protein and the potential signaling pathway connecting Gene 33, the small GTPase Cdc42, and JNK
[4]. The laboratories of Oreste Segotto and Axel Ullrich independently discovered the interaction between rat Gene 33 with ErbB2 and Mig6 with EGFR, respectively, using the yeast two-hybrid system
[15][16]. The interactions were found to inhibit the signaling pathways and fibroblast transformation mediated by these receptors
[15][16]. These studies also showed that Gene 33 can also be induced by the activation of these receptors, establishing that Gene 33 is a potential feedback inhibitor of the signaling pathway mediated by these receptors. The Segotto group renamed Gene 33 as
receptor-
associated
late
transducer (RALT). The official name given later by the Human Genome Organization to this gene is
ErbB
receptor
feedback
inhibitor
1 (
ERRFI1). This review will refer to this protein using its original common name “Gene 33” and the gene encoding it using its official name
ERRFI1 (
Errfi1 for the rodent gene).
The involvement of Gene 33 in the signaling of the ErbB family receptor tyrosine kinases (RTKs) sparked intense interest in this then little-known protein and led to a series of studies that solidified its role in the ErbB receptor signaling pathway
[11][17][18][19][20][21][22][23][24][25][26]. Although the regulation of ErbB receptors appears to be the most prominent function of Gene 33, its involvement in other signaling pathways has also been revealed. The biological roles of Gene 33 in various pathophysiological processes have become clearer as well. Although Gene 33 has long been regarded as an exclusively cytoplasmic protein, recent evidence showed that a fraction of it is localized in the nucleus and associated with chromatin. This nuclear/chromatin fraction of Gene 33 has been shown to modulate the DNA damage response in response to genotoxic stresses
[27][28]. These new findings significantly expand the functional profile of this protein. Several excellent and focused review articles on Gene 33 have been published in the past, with main emphases on its association with the ErbB family RTKs and its role in cancer
[29][30][31][32].
2. Gene 33 and Human Diseases
2.1. Gene 33 in Cancer
The involvement of Gene 33 in the signaling of receptor tyrosine kinases of the EGFR family prompted immediate interest in its potential role in cancer, as these receptors are heavily involved in various types of human cancer
[33]. The function of Gene 33 as an inhibitor of these receptors suggests a potential tumor suppressive role of this protein. Other known functions of Gene 33, including the proapoptotic and the nuclear function of Gene 33, are also largely in line with this notion. Indeed,
Errfi1-null mice develop neoplasms in multiple tissues
[20]. In humans,
ERRFI1 is located in chromosome 1p36, a locus long believed to contain multiple putative tumor suppressor genes and frequently altered in many cancers
[34]. These data support a general role of Gene 33 in human cancer as a tumor suppressor.
2.2. Gene 33 in Diabetes
Errfi1 was shown initially by the laboratory of Joseph Messina to be an insulin-inducible gene in H4 hepatocarcinoma cells soon after its discovery
[35]. Follow-up studies confirmed this observation
[2][8][36][37][35]. As in other settings, the induction of Gene 33 by insulin is MEK-ERK dependent
[37]. These findings suggest a potential role of Gene 33 in the development of diabetes. Supporting this notion, Gene 33 expression is strongly induced in a murine model for diabetic nephropathy and was proposed to mediate diabetic renal hypertrophy
[4]. Furthermore, a study based on a Korean population revealed a single nucleotide polymorphism (SNP) in the third intron of the
ERRFI1 gene (+808(T/G)) that is negatively associated with diabetic nephropathy
[38]. This SNP is believed to negatively regulate the expression of Gene 33
[38].
Errfi1 haploinsufficiency protects mice from streptozotocin-induced diabetes
[39]. This prodiabetic function of Gene 33 appears to be a result of the ability of Gene 33 to promote DNA damage-induced apoptotic death of pancreatic β cells in response to proinflammatory cytokines and ER stress
[40][39]. Gene 33 may also mediate glucocorticoid-induced insulin resistance and diabetes by suppressing β cell proliferation via inhibition of EGFR signaling
[41]. In contrast, mice with liver-specific knockout of
Errfi1 exhibit hyperglycemia as a result of hepatic insulin resistance
[42]. Deletion of
Errfi1 increases EGFR signaling, mTOR activity, JNK activity, and IRS-1 phosphorylation at serine 307
[42]. On the other hand, hepatic expression of glucokinase, glucose-6-phosphatase, and phosphoenolpyruvate carboxykinase 1 is reduced after
Errfi1 ablation
[42]. Interestingly, another study showed that liver-specific knockout of
Errfi1 in mice leads to fatty liver, fasting hyperglycemia, and hypercholesterolemia but lower body weight and higher insulin sensitivity
[43]. The hypercholesterolemia in the knockout mice is apparently a result of increased EGFR activation, as inhibition of EGFR with TKI reduces hypercholesterolemia
[44]. These findings highlight a rather complex role of Gene 33 in diabetes and metabolic syndrome, and possible differential roles in conditions mimicking type 1 and type 2 diabetes.
2.3. Gene 33 in Cardiovascular Diseases
ErbB2 signaling is well established to promote cardiomyocyte survival while inhibition of it leads to cardiomyocyte apoptosis and dilated heart failure
[45][46][47][48][49]. Gene 33 overexpression using the adenoviral vector leads to strong apoptosis of rat neonatal myocytes as a result of inhibition of the antiapoptotic AKT and ERK activity downstream of ErbB family tyrosine kinases
[17]. Gene 33 is induced in rat neonatal myocytes by hypoxia and hypoxia/reoxygenation and in turn promotes apoptosis
[17]. Moreover, Gene 33 is strikingly induced in the murine cardiac tissue suffering experimentally induced myocardial infarction or ischemia and ischemia/reperfusion
[17]. These findings point to a significant role of Gene 33 in the loss of cardiomyocytes during ischemia induced by myocardial infarction by promoting cardiomyocyte apoptosis.
On the other hand, constitutive cardiac-specific ectopic expression of Gene 33 in mouse heart attenuates both hypertrophic and inflammatory responses and helps preserve cardiac functions
[50]. This is accomplished through inhibition of the angiotensin II and isoproterenol-stimulated vasoactive signaling pathways, which are known to transactivate EGFR
[51][50]. Unlike in the case of ischemic stress, transgenic expression of Gene 33 does not cause significant cardiomyocyte apoptosis in mice, highlighting the differential role of Gene 33 in acute and chronic cardiac conditions
[17][50].
Using smooth muscle-specific
Errfi1 knockout mice and the cellular model, Lee et al. showed that Gene 33 inhibits EGF-dependent smooth muscle proliferation and migration that lead to neointimal hyperplasia, thereby potentially inhibiting atherosclerosis
[52].
2.4. Gene 33 in Other Diseases
Gene 33 has been documented to promote intervertebral disk degeneration, in which miR-2355 is upregulated and suppresses the expression of Gene 33
[53]. Gene 33 in turn inhibits EGFR-mediated proliferation of intervertebral disc cells, particularly nucleus pulposus cells
[53]. Microarray analyses detected upregulation of
ERRFI1 in the central nervous system after acute infection by pseudorabies virus, suggesting a potential involvement of Gene 33 in the response of the central nervous system to viral infection
[54]. Downregulation of
Errfi1 was observed in the liver of mice subjected to disrupted growth hormone and insulin-like growth factor-1 signaling as well as caloric restriction, suggesting a potential involvement of Gene 33 in dwarfism and longevity
[55]. Interestingly, a reduced plasma level of Gene 33 has been shown to be associated with children with autism
[56].
A recent integrated analysis of public genomic data revealed a potential association between
ERRFI1 with the risk of psoriasis, a chronic inflammatory skin condition
[57]. An enhancer variant in the chromosome 1p36.23 region and within the enhancer of
ERRFI1 is associated with increased susceptibility for psoriasis. The variant may render the
ERRFI1 enhancer less able to interact with the AP-1 complex at the
ERRFI1 promoter, thereby destabilizing the expression of the gene
[57]. Interestingly, psoriasis has been shown to be associated with nonalcoholic fatty liver disease, type 2 diabetes, and metabolic syndrome
[58]. These findings provide further support to the involvement of Gene 33 in diabetes and metabolic syndrome.
Table 1 summarizes the known association of Gene 33 with human diseases.
Table 1. Gene 33 and human diseases.
| Function in Cancer |
Cancer Type |
Reference |
| Suppressor |
Lung cancer |
[20][28][59][60][61][62][63][64][65] |
| Endometrial cancer |
[66][67][68][69][70] |
| Glioma |
[25][71][72][73] |
| Thyroid cancer |
[74][75][76][77] |
| Liver cancer |
[26][78] |
| Suppressor or promotor |
Breast cancer |
[24][79][80][81] |
| Skin cancer |
[20][82][83] |
| Marker for liver metastasis |
Colorectal cancer |
[84] |
| Function in other diseases |
Disease |
Reference |
| Suppressor |
Cholangiocarcinoma |
[85] |
| promotor |
Intervertebral disk degeneration |
[53] |
| Suppressor or promotor |
Diabetes |
[4][41][40][38][39][42][43] |
| Suppressor or promoter |
Cardiovascular diseases |
[17][50][52] |
| Positive correlation |
Dwarfism and longevity |
[55] |
| Negative correlation |
Psoriasis |
[57] |
| Negative correlation |
Autism |
[56] |
3. Conclusions and Perspectives
Our understanding of Gene 33 has come a long way since its discovery more than 30 years ago. The nature of this protein as an adapter/scaffold and the relative late appearance in the evolution suggest that its main functions are likely to coordinate multiple cellular pathways in response to the increased biological complexity of higher metazoans. The functions of Gene 33 discovered to date appear to support this notion. It would not be surprising that more functions of Gene 33 will be revealed along this line of reasoning. Recent evidence on its nuclear and chromatin-associated functions in DDR and DNA repair points to a new direction in searching for additional functions of this protein. Although Gene 33 may not be one of the core regulators of these biological processes, its importance in pathophysiological processes should not be overlooked. In fact, many biological processes, chronic pathological conditions in particular, are strongly associated with seemingly weak biological alterations rather than overt biological defects with severe consequences, e.g., early mortality. Therefore, the study of proteins, such as Gene 33, is important for better understanding of these chronic conditions, which could lead to novel therapeutic strategies.
For instance, the nuclear function of Gene 33 on DDR may contribute to carcinogenesis induced by genotoxic agents with environmental or occupational relevance, such as hexavalent chromium, by affecting Cr(VI)-induced genomic instability. Gene 33 dysregulation may also facilitate spontaneous gene mutations that lead to genomic instability and carcinogenesis. In diabetes, abnormal function of nuclear Gene 33 could contribute to chronic loss of β cells through dysregulation of cell cycle checkpoint control at the G2/M phase. A similar mechanism could be involved in psoriasis, where abnormal proliferation of skin cells contributes to the symptom of the disease. The nuclear function of Gene 33 adds additional complexity to the already complex functional profile of Gene 33. Further investigation into the role of nuclear Gene 33 in chronic human diseases is clearly needed and potentially fruitful.

 Figure 1. Comparison of linear structures of Gene 33 and ACK-1. SH3: Src homology 3 domain, CRIB: Cdc42/Rac interactive and binding domain. AH: Ack homology domain.
Figure 1. Comparison of linear structures of Gene 33 and ACK-1. SH3: Src homology 3 domain, CRIB: Cdc42/Rac interactive and binding domain. AH: Ack homology domain.




