
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eliana Alves | + 2004 word(s) | 2004 | 2021-06-30 05:43:33 | | | |
| 2 | Peter Tang | Meta information modification | 2004 | 2021-07-01 04:53:50 | | |
Video Upload Options
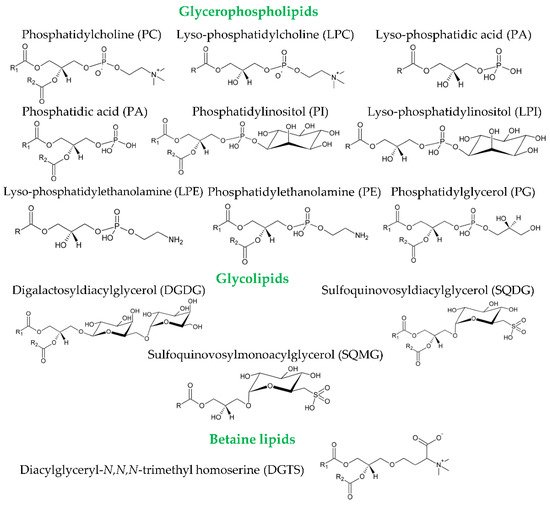
Polar lipids are minor components of olives and olive oil and include a myriad of molecules such as phospholipids and glycolipids.
1. Introduction
2. Identification of Polar Lipids from Olives, Olive Oil, and Their Industrial By-Products
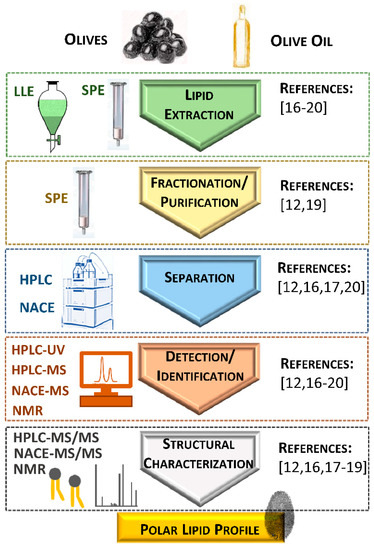
|
Reference |
Sampling |
Analysis |
Polar Lipid Classes |
||
|---|---|---|---|---|---|
|
Type of Sample |
Amount of Sample |
Extraction |
Method |
||
|
[20] |
Olive fruit and olive oil from varieties Carolea and Ottobratica, both from Calabria region (Italy) |
Olive fruit (250 g); olive oil (10 mL) |
Glycosidic fraction in olive fruit: ethanol and “charcoal method”; glycosidic fraction in the aqueous phase of olive oil: ethyl acetate/dichloromethane (1:1 by volume) and water |
HPLC-UV (µ-Bondapak C18 column) |
DGDG |
|
[16] |
Tunisian commercial olive oil |
Not said |
Modified Bligh and Dyer method |
HPLC-MS/MS (diol column) |
PG (63%), PA (12%), PI (11%), PE (9%), PC (5%) |
|
[18] |
Greek virgin olive oil, refined olive oil and olive pomace oil from local cooperatives (7 regions and 5 cultivars) |
100 g |
According to Galanos and Kapoulas (1962) |
31P-NMR |
PA, lyso-PA, lyso-PI, PI, PG (PG only in pomace oil), PC and PE (these two only in virgin olive oil). |
|
[17] |
Olive pulp and olive stone from Spanish Arbequina variety from three geographical regions (Córdoba, Jaén, and Toledo) and two Spanish varieties (Empeltre and Lechín de Sevilla) from the same region (Córdoba); commercial monovarietal extra virgin olive oil from Arbequina variety |
Olive pulp or stone (2.5 g); olive oil (50 g) |
PL from olive pulp and stone: modified Folch method; PL from olive oil: LLE according to Galanos and Kapoulas (1962) |
NACE-ESI-MS and MS/MS |
Olives (stone and pulp studied independently): PA (54−82%), PE (4−16%), PC (3−9%), lyso-PE (1.3−18%), PI (4.4−8%), PG (3.7−6.3%), and lyso-PA (0.1−0.2%). |
|
Olive oil: PE (42%), PG (38%), PC (15%), lyso-PE (4.5%), and lyso-PA (0.2%) |
|||||
|
[19] |
Italian olive oil blend (Leccino, Frantoio and Picholine varieties) from a local mill of Emilia Romagna region (Italy) |
100 g for LLE; 40 g for SPE |
LLE according to Galanos and Kapoulas (1962) followed by SPE (diol and silica). PL eluted with methanol and chloroform/methanol/water (3:5:2 by volume) |
HPLC–ESI-qTOF-MS (HILIC column) |
Diol extracted veiled extra virgin olive oil (mg kg−1): lyso-PA (4.23), lyso-PC (1.21), PI (1.03), PC (0.90), PA (0.81), PG (0.07). Crystallized veiled virgin olive oil (mg kg−1): lyso-PA (1.15), lyso-PC (0.87), PC (0.74), PI (0.48), PA (0.14) |
|
[12] |
Portuguese commercial extra virgin and virgin olive oils |
1 g |
PL extracted by SPE (aminopropyl columns) and eluted with acetonitrile: ammonium hydroxide (95:5 by volume) |
HPLC-ESI-ion trap-MS/MS (HILIC column) |
PA, PE, PG, PC, PI, SQDG, SQMG, DGTS |
Legend: DGDG, digalactosyldiacylglycerol; DGTS, diacylglyceryl-N,N,N-trimethylhomoserine; HILIC, hydrophilic interaction liquid chromatography; HILIC-ESI-MS/MS, hydrophilic interaction liquid chromatography coupled to electrospray ionization tandem mass spectrometry; HPLC, high-performance liquid chromatography; HPLC-ESI-qTOF-MS, high-performance liquid chromatography coupled to electrospray ionization-quadrupole time-of-flight mass spectrometry; HPLC-UV, high-performance liquid chromatography with ultraviolet detector; HPLC-MS/MS, high-performance liquid-chromatography coupled to tandem mass spectrometry; LLE, liquid/liquid extraction; MS/MS, tandem mass spectrometry; NACE-ESI-MS, non-aqueous capillary electrophoresis coupled to electrospray ionization mass spectrometry; NMR, nuclear magnetic resonance; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PL, polar lipid; SPE, solid-phase extraction; SQDG, sulfoquinovosyldiacylglycerol; SQMG, sulfoquinovosylmonoacylglycerol.
3. Potential Biotechnological Uses of Polar Lipids from Olives’ and Olive Oil’s Industrial By-Products
References
- International Olive Council. World Olive Oil Figures. Available online: (accessed on 18 December 2017).
- Boskou, D. 1-Olive oil: Properties and processing for use in food. In Specialty Oils and Fats in Food and Nutrition, 1st ed.; Talbot, G., Ed.; Woodhead: Cambridge, UK, 2015; pp. 3–38. ISBN 9781782423768.
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989–2007.
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules 2017, 22, 1833.
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84.
- Aparicio, R.; Conte, L.S.; Fiebig, H.-J. Chapter 16. Olive oil authentication. In Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 589–5654. ISBN 978-1-4614-7777-8.
- Perri, E.; Benincasa, C.; Muzzalupo, I. Chapter 13. Olive oil traceability. In Olive Germplasm-the Olive Cultivation, Table Olive and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; InTechOpen: London, UK, 2012; pp. 265–286. ISBN 978-953-51-0883-2.
- Montealegre, C.; Marina Alegre, M.L.; García-Ruiz, C. Traceability markers to the botanical origin in olive oils. J. Agric. Food Chem. 2010, 58, 28–38.
- Aparicio, R.; Morales, M.T.; Aparicio-Ruiz, R.; Tena, N.; García-González, D.L. Authenticity of olive oil: Mapping and comparing official methods and promising alternatives. Food Res. Int. 2013, 54, 2025–2038.
- Gallina Toschi, T.; Bendini, A.; Lozano-Sánchez, J.; Segura-Carretero, A.; Conte, L. Misdescription of edible oils: Flowcharts of analytical choices in a forensic view. Eur. J. Lipid Sci. Technol. 2013, 115, 1205–1223.
- Bajoub, A.; Bendini, A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Olive oil authentication: A comparative analysis of regulatory frameworks with especial emphasis on quality and authenticity indices, and recent analytical techniques developed for their assessment. A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 832–857.
- Alves, E.; Melo, T.; Rey, F.; Moreira, A.S.; Domingues, P.; Domingues, M.R. Polar lipid profiling of olive oils as a useful tool in helping to decipher their unique fingerprint. LWT Food Sci. Technol. 2016, 74, 371–377.
- Calvano, C.D.; De Ceglie, C.; D’Accolti, L.; Zambonin, C.G. MALDI-TOF mass spectrometry detection of extra-virgin olive oil adulteration with hazelnut oil by analysis of phospholipids using an ionic liquid as matrix and extraction solvent. Food Chem. 2012, 134, 1192–1198.
- Karantonis, H.C.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic lipid minor constituents from vegetable oils. Comparison between olive oils and others. J. Agric. Food Chem. 2002, 50, 1150–1160.
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic and antiatherosclerotic properties of olive oil and olive pomace polar extracts in rabbits. Med. Inflamm. 2007, 2007, 11.
- Boukhchina, S.; Sebai, K.; Cherif, A.; Kallel, H.; Mayer, P.M. Identification of glycerophospholipids in rapeseed, olive, almond, and sunflower oils by LC-MS and LC-MS-MS. Can. J. Chem. 2004, 82, 1210–1215.
- Montealegre, C.; Sanchez-Hernandez, L.; Crego, A.; Marina, M. Determination and characterization of glycerophospholipids in olive fruit and oil by nonaqueous capillary electrophoresis with electrospray-mass spectrometric detection. J. Agric. Food Chem. 2013, 61, 1823–1832.
- Hatzakis, E.; Koidis, A.; Boskou, D.; Dais, P. Determination of phospholipids in olive oil by 31P-NMR spectroscopy. J. Agric. Food Chem. 2008, 56, 6232–6240.
- Verardo, V.; Gómez-Caravaca, A.; Montealegre, C.; Segura-Carretero, A.; Caboni, M.; Fernández-Gutiérrez, A.; Bendini, A. Optimization of a solid phase extraction method and hydrophilic interaction liquid chromatography coupled to mass spectrometry for the determination of phospholipids in virgin olive oil. Food Res. Int. 2013, 54, 2083–2090.
- Bianco, A.; Mazzei, R.A.; Melchioni, C.; Scarpati, M.L.; Romeo, G.; Uccella, N. Microcomponents of olive oil. Part II: Digalactosyldiacylglycerols from Olea europaea. Food Chem. 1998, 62, 343–346.
- Scrimgeour, C.M.; Harwood, J.L. Chapter 1: Fatty acid and lipid structure. In The Lipid Handbook, 3rd ed.; Gunstone, F.D., Harwood, J.L., Dijkstra, A.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–36. ISBN 978-0849396885.
- Dembitsky, V.M. Betaine ether-linked glycerolipids: Chemistry and biology. Prog. Lipid Res. 1996, 35, 1–51.
- Moussaoui, R.; Labbaci, W.; Hemar, N.; Youyou, A.; Amir, Y. Physico-chemical characteristics of oils extracted from three compartments of the olive fruit (pulp, endocarp and seed) of variety chemlal cultivated in Kabylia (Algeria). J. Food Agric. Environ. 2008, 6, 52–55.
- Koidis, A.; Boskou, D. The contents of proteins and phospholipids in cloudy (veiled) virgin olive oils. Eur. J. Lipid Sci. Technol. 2006, 108, 323–328.
- Nasopoulou, C.; Zabetakis, I. Agricultural and aquacultural potential of olive pomace a review. J. Agric. Sci. 2013, 5, 116–127.
- Caputo, A.; Morone, G.; Di Napoli, M.A.; Rufrano, D.; Sabia, E.; Paladino, F.; Sepe, L.; Claps, S. Effect of destoned olive cake on the aromatic profile of cows’ milk and dairy products: Comparison of two techniques for the headspace aroma profile analysis. Ital. J. Agron. 2015, 10, 15–20.
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary supplementation with dried olive pomace in dairy cows modifies the composition of fatty acids and the aromatic profile in milk and related cheese. J. Dairy Sci. 2017, 100, 8658–8669.
- Cibik, M.; Keles, G. Effect of stoned olive cake on milk yield and composition of dairy cows. Revue Méd. Vét. 2016, 167, 154–158.
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian-Australas. J. Anim. Sci. 2013, 26, 971–980.
- Vargas-Bello-Pérez, E.; Vera, R.R.; Aguilar, C.; Lira, R.; Peña, I.; Fernández, J. Feeding olive cake to ewes improves fatty acid profile of milk and cheese. Anim. Feed Sci. Technol. 2013, 184, 94–99.
- Karantonis, H.C.; Tsantila, N.; Stamatakis, G.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S.; Demopoulos, C.A. Bioactive polar lipids in olive oil, pomace and waste byproducts. J. Food Biochem. 2008, 32, 443–459.
- Nasopoulou, C.; Smith, T.; Detopoulou, M.; Tsikrika, C.; Papaharisis, L.; Barkas, D.; Zabetakis, I. Structural elucidation of olive pomace fed sea bass (Dicentrarchus labrax) polar lipids with cardioprotective activities. Food Chem. 2014, 145, 1097–1105.
- Are Olive Seeds the Next Superfood? Available online: (accessed on 7 January 2018).
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788.
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107.




