Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Iwona Kwiatkowska | + 3829 word(s) | 3829 | 2021-06-04 07:55:32 | | | |
| 2 | Peter Tang | Meta information modification | 3829 | 2021-06-16 04:30:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kwiatkowska, I. TDO2/IDO2 in Cancer Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/10851 (accessed on 08 February 2026).
Kwiatkowska I. TDO2/IDO2 in Cancer Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/10851. Accessed February 08, 2026.
Kwiatkowska, Iwona. "TDO2/IDO2 in Cancer Development" Encyclopedia, https://encyclopedia.pub/entry/10851 (accessed February 08, 2026).
Kwiatkowska, I. (2021, June 15). TDO2/IDO2 in Cancer Development. In Encyclopedia. https://encyclopedia.pub/entry/10851
Kwiatkowska, Iwona. "TDO2/IDO2 in Cancer Development." Encyclopedia. Web. 15 June, 2021.
Copy Citation
Elements involved in the tryptophan metabolism pathway and its derivatives are considered factors that play a wide role in silencing the immune system. However, it seems that those agents contribute to tumorigenesis through a direct impact on cancer cells.
kynurenine pathway
tryptophan
epithelial–mesenchymal transition
carcinogenesis
circulating tumor cells
1. Introduction
The intensive development of science in the field of immuno-oncology gives hope for a thorough understanding of the changes taking place in the body during the development of neoplasms. One of the pathways that has been particularly strongly studied in this context is the metabolism of tryptophan (TRP). Efforts are being made to assign a pro- or anti-tumor role to individual elements of this metabolic pathway. However, the complexity and multiplicity of intertwined processes still leave more questions than answers and represent an open field for future research.
2. Mechanisms Involved in Immune Evasion
For many years, the role of the immune system in cancer development has been under detailed scientific investigation. It is known that immune cells have an ability to recognize developing malignant cells. On the other hand, the enhanced activity of regulatory Tcells (Treg) leads to a decreased immune response and facilitates tumor growth. Moreover, cancer cells secrete factors that favor immunosuppressive microenvironment development, which further enables them to avoid the immune mechanism. This phenomenon is called “immunoediting” and is based on the hypothesis, that the immune system can be both—a tumor suppressor and promotor. It is divided into three phases—elimination, equilibrium, and escape. The first stage is based on observations that cancer cells express specific antigens that are recognized by dendritic cells (DCs) and presented to lymphocytes T, which in turn eliminate pathological cells through cytotoxic mechanisms [1]. In this step, tumor cells can be recognized also by macrophages and natural killers, which altogether leads to Tcell activation [2]. If the immune system eliminates all of the abnormal cells, the whole process of tumor development stops. However, some growing cells seem to be resistant to host immune mechanisms, which leads to the second phase—equilibrium. In this step, dynamic processes remain in balance, but cancer cells gain features that allow them to avoid immune recognition with the following destruction. Factors such as IL-12, IL-23 (interleukin 12, 23) and the elements of adaptive immune response keep tumor cells in a silent state [3]. However, if malignant cells obtain superiority, they progress to the third phase of immunoediting—escape—and become clinically apparent. To reach this state, cancer cells expand multiple mechanisms which facilitate their immunological evasion. One of them is based on a decreased expression of MHC-I (major histocompatibility complex, class I), with the following disturbances in antigen expression or presentation. This leads to inhibition in an antigenicity and moderates a recognition of tumor cells by immune factors. Another way to silence host defense response toward developing malignant cells is the production of molecules, which serve as immune inhibitors. Among them, programmed death-ligand-1 (PD-L1) is one of the most studied and well-known molecules, which already serves as a pharmacological target point in clinical practice. This ligand, after binding to its receptor PD-1 (programmed cell death protein 1) on immune cells, exerts multiple effects on them. Enhanced conversion of CD4+ Tcells into immunosuppressive Treg, decreased cytotoxicity of CD8+ Tcells, reprogramming macrophages into M2 subtypes, which inhibit immunity, are among well-studied effects acquired after PD-L1/PD-1 pathway activation [4][5]. The third and the most complex mechanism triggered by cancer cells for immune evasion is the excretion of prosurvival factors and molecules, which enable the production of an immunosuppressive microenvironment. It can be obtained by the secretion of cytokines with the following recruitment of Treg and MDSCs (myeloid-derived suppressor cells), and changes in amino-acids metabolism. Arginine, glutamine, leucine, and tryptophan are among those which are now under investigation and establishment of their function will help to better understand the biology of cancer [6][7][8]. Due to the broad role of each of the listed amino-acids, it is not possible to describe them in detail in one manuscript. For this reason, the given paper focuses selectively on one of them—TRP—and its metabolism via the kynurenine pathway (KP) as a factor enhancing tumor development.
3. Tryptophan Metabolism and Its Modulators
Tryptophan, an endogenous amino-acid essential for proper organism development in the course of further metabolic transformations, is converted into indole, with the participation of intestinal microflora, into serotonin (5-HT) under the influence of TRP hydroxylase 2 enzymes, and in the highest level is metabolized through kynurenine pathway [9]. The latter involved two isoforms of indoleamine oxidases (IDO1, IDO2) and tryptophan 2,3-oxidase (TDO2) which are rate-limiting enzymes, that degrade TRP into kynurenine (Kyn). The next steps lead to the transformation of Kyn into kynurenic acid (KYNA), 3-hydroxykynurenine (3-HKYN), antranilic acid (AA) and further production of xanturenic (XA), picolinic (PA) and quinolinic (QUIN) acids. In the final step, active NAD+ (nicotinamide adenine dinucleotide) arises. Most TRP metabolites are active and exert a multiple and differentiated role in cancer development, which is described in detail below. Here, it should be emphasized that malignant cells can produce individual elements of KP, as well as factors that enhance the activity of this pathway. IDO1 expression is observed in almost all human tissues and its expression upraises with age, IDO2 at the highest level can be observed in the liver, epididymis and brain, and TDO2 except the liver and brain, can be found in the placenta [10]. All of them may be detected in different types of cancers with various severity. Those three enzymes, as rate-limiting factors of TRP metabolism, gained the greatest scientific interest and are now under intense development as a potential therapeutic goal in cancer immunotherapy. Understanding which factors take part in controlling KP elements can shed a light on new therapeutic strategies in oncology. The main molecules involved in IDO-1 activity regulation are proinflammatory agents, i.e., lipopolysaccharides, pathogen-associated molecular patterns, TGF-β (transforming growth factor-beta), and IFN-γ (interferon-gamma) at the forefront. Chronic inflammation is a hallmark of cancer, so the involvement of the aforementioned factors in both processes, i.e., inflammation and expression of TRP catabolizing enzymes, suggests that kynurenine pathway elements play a role in carcinogenesis. An additional argument indicating the involvement of KP in the development of tumorigenesis is an observed correlation between the presence of the known proto-oncogene MYC and overexpression of tryptophan transporters and its increased intracellular transport. Furthermore, the level of KP enzymes is significantly higher in the presence of MYC, when compared to a knock-out cell line [11]. Another oncogene, whose activity influences IDO1 regulation, is the c-KIT proto-oncogene. Balachandran et al. showed that the inhibition of KIT signaling significantly decreases IDO1 activity, confirming the role of this oncogene in KP regulation [12]. Conversely, the high activity of tumor suppressor protein Bin-1 was connected with low IDO1 expression and better prognosis for patients [13], which additionally links the TRP metabolite pathway with tumor development.
An expression of TDO2 is controlled by corticosteroids, and it was shown that cortisol increases gene transcription of this protein [14]. Other hormones, such as estrogen and testosterone, seem to have no impact on TDO expression [15][16]. Still, TRP itself induces TDO2 expression and thus its high dietary intake is a stimulator for this protein. Moreover, in mice models, a high-fat diet was indicated as a factor uprising liver TDO2 level [17]. This may point to a potential role of a balanced diet in cancer prevention. In regards to cancer development and KP regulation, the role of the active form of vitamin B6 (PLP) cannot be overlooked, as its low serum level is correlated with a higher risk of disease occurrence. PLP is a cofactor of KP enzymes, i.e., kynureninase and kynurenine aminotraspherases, which take part in the transformation of Kyn to AA and HK to HAA. Therefore, low B6 supply and its systemic deficiency lead to the accumulation of procancerous metabolites [18]. The gathered information shows how complex and multifactorial the tryptophan metabolism pathway is. Moreover, most of the formed metabolites enhance cancerogenesis, but some of them seem to play a protective role, which makes the whole pathway more challenging to use as a therapeutic target. The further part of this manuscript describes the known aspects of KP elements in cancerogenesis modulation (Figure 1).
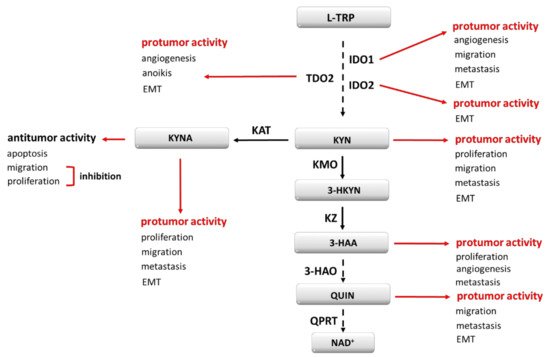
Figure 1. Kynurenine pathway elements as cancerogenesis modulators. EMT—epithelial-to-mesenchymal transition; 3-HAA—3-hydroxyanthranilic acid; 3-HAO—3-hydroxyanthranilate-3,4-dioxygenase; 3-HKYN—3-hydroxykynurenine; IDO1, IDO2—indoleamine oxidase 1, 2; KAT—kynurenine aminotransferase; KMO—kynurenine 3-monooxygenase; KYNA—kynurenine acid; KYN—kynurenine; KZ—kynureninase; L-TRP—tryptophan; NAD+—nicotinamide adenine dinucleotide; QPRT—quinolinic acid phosphoribosyltransferase; QUIN—quinolinic acid; TDO2–tryptophan 2,3-oxidase.
4. IDO1 and Its Role in Cancer Development
IDO1, the best-studied enzyme of all KP, seems to play one of the most complex roles in cancerogenesis. TRP depletion in local milieu IDO1 affects immunological cells and silences immune response. The activation of GCN-2 kinase (general control nonderepressible 2 kinase) and the inhibition of mTOR (mechanistic target of rapamycin) pathway lead to an immunosuppressive phenotype and facilitated immunosurveillance escape by cancer cells. It has been discovered that IDO1 has a direct impact on malignant cells and enhances tumorigenesis by increasing angiogenesis and metastasis. Moreover, this enzyme conditions metabolite production which contributes to oncogenesis either through AhR (aryl hydrocarbon receptor) activation or in other mechanisms described below. Due to that fact, IDO1, as a pharmacological target, has a high potential in oncology and its inhibition gives an opportunity for increased therapeutic successes. The depletion of TRP affects dendritic cells and two populations of lymphocyte T—immunosuppressive regulatory Tcells (Treg) and cytotoxic lymphocyte T (Tc). TRP deficiency, through the activation of GNC-2, is a signal enhancing the expression of inhibitory receptors (ILT3, ILT4) in DCs [19]. Those cells, as antigen-presenting cells (APCs) to lymphocytes T, as well take part in the differentiation of naïve Tcells in chosen subpopulations. The overexpression of ILTs, especially ILT3 and ILT4 on DCs, induces the differentiation of CD8+ and CD4+ Tcells into Treg [20]. Additionally, in CD4+ Th cells, the presence of ILTs enhances their anergy, thus inhibits the antitumor role. A high expression of ILTs essential for Treg induction [21] altogether favors the immunosuppressive phenotype. In general, those receptors are suspected to be a key factor inducing tolerogenicity [22] thus developing immunological tolerance in host organisms. The role of immunoglobulin-like transcript receptors in cancer development has been reported previously [23]. In few cancer types, such as breast, colorectal, non-small cell lung cancer, and renal cell carcinoma, high expression of those proteins was generally connected with more advanced stages of the disease, more often occurring metastasis, and poor prognosis for patients. The mentioned research points on the activation of ERK1/2 (extracellular signal-regulated kinases) signaling pathway, enhanced epithelial-to-mesenchymal transition (EMT), and increase in VEGF (vascular endothelial growth factor) level, which altogether contributes to augmented motility, angiogenesis, and invasiveness of cancer cells [24][25][26][27]. The gathered information shows that TRP depletion affects immune cells, which has a direct impact on cancer cells, their increased motility, metastasis potential and results in patients’ worse prognosis and overall survival. Another structure affected by GCN-2 activation exerted by IDO-1-induced TRP depletion is ζ-chain of T-cell receptor (TCR) in CD8+ Tcells. This structure is a key agent which conditions the occurrence of signaling from TCR [28], with the following activation of CD8+ and their full antitumor immune response [29]. Therefore, its downregulation in case of non-sufficient TRP level interrupts the cascade of events with the following impairment in lymphocyte proliferation and cytokine production [30]. To fully understand how the downregulation of the zeta chain in TCR affects tumor development, it is necessary to describe the role of CD8+ lymphocytes in this process. Those cells are at the highest level responsible for the direct killing of tumor cells through the secretion of cell membrane perforating molecules, i.e., cathepsin C, perforin, granzymes. The second mechanism, induced by CD8+ Tcells, which leads to cell apoptosis, is their expression of the Fas ligand, which, after binding to its receptor on the targeted cell, induces caspases and endonucleases leading to DNA impairment [31]. Previously, it was shown that a low number of CD8+ Tcells correlates with enhanced tumor growth and poor prognosis [32][33]. Detailed research points to a zeta chain as a key factor mediating antitumor response. In the case of oral cancer and Hodgkin’s Disease, a low level of the zeta chain in TCR in peripheral Tcells is correlated with a more advanced stage of disease [34][35]. Moreover, enhancing TCR signaling by binding immunoglobulin superfamily member 4 (IGSF4) to the zeta chain significantly decreased tumor size and weight in murine models with implanted melanoma cells and reduces the occurrence of metastatic colonies. [36]. Still, the high activity of IDO1, and thus a decreased TRP level leads to abnormal activity of immunosuppressive Tregs, whose pro-tumorigenic activity is based on crosstalk with other immune cells, as well as on a direct impact on cancer cells. Those cells express CD73 and CD39 endonucleotidases, which take part in adenosine production [37]. The overexpression of the latter was connected to an increased number of occurring liver metastasis in colorectal cancer (CRC) in the murine model and correlates with the worst prognosis and a poor outcome in patients [38]. Adenosine, whose production from ATP and AMP is enhanced in the presence of Treg, affects tumor development by activating PI3K/Akt/mTOR pathway and upregulating metalloproteinases that stimulate invasiveness and migration capacity of malignant cells [39]. Additionally, the whole loop leading to adenosine excretion leads to angiogenesis, caused by an intensified production of VEGF [40]. Besides the direct act on processes promoting oncogenesis, an elevated number of Tregs, and thus IDO1 activity, leads to the development of the immunosuppressive tumor microenvironment. High activity of Tregs limits interleukin 2 (IL-2) production, with the following CD8+ lymphocyte inhibition [41]. Other cells from the immune system, whose activity is at least in part controlled by Tregs are cancer-associated fibroblasts (CAFs), macrophage type 2 (M2) cells, regulatory B cells (Bregs), and myeloid-derived suppressor cells [42]. It should be emphasized here that the latter can secrete IDO1 [43], which loops the course of events even more. All of them are considered to be tumor promoters, their activity being increased under the impact of Treg. The outcomes of the experiments confirm the crucial role of Treg in cancerogenesis. In a few cancer types, i.e., gastric, breast, renal a higher intratumoral Treg level correlates with a worse prognosis [44][45][46]. Additionally, it was reported that high Treg activity contributes to chemoresistance [47][48]. On the other hand, clinical reports indicate the inhibition of inflammatory response by Treg, which in a further perspective leads to a decreased level of occurring tumor [49]. Taking into account the double role of Treg in cancer development, and IDO1 effect on both subpopulations of lymphocytes—the downregulation of CD8+ cytotoxic Tcells and the upregulation of immunosuppressive Treg it seems reasonable to focus on the ratio between these lines as the most proper prognostic factor in the context of IDO1 activity [50]. The outcomes from oncological patient samples confirm that a high CD8+/Treg ratio, and hence a low IDO1 activity, is associated with a more favorable prognosis [51][52][53]. The described effects are the results obtained by the activation of GCN-2 kinase. However, it was mentioned before that TRP depletion affects mTOR kinase, with its inhibition. This results in a decreased number of cytotoxic and helper T cells and an increased number of immunosuppressive Treg in the general population. Besides an indirect impact of IDO1 on cancer cells, it excretes its own direct effect. In bladder cancer cells, IDO1 inhibition leads to a limited colony formation, an increased E-cadherin expression with a concomitantly reduced N-cadherin and vimentin presence. This in total points to IDO1 being a promoter of epithelial-to-mesenchymal transition and a factor that facilitates a gain in motility capacity by cancer cells. Moreover, in the same experiment, the authors show a reduced ability for tubule formation by HVUECs and thus decreased angiogenesis after silencing IDO1 [54]. Those results are in the line with the results obtained by Pan et al., who reported decreased cell invasiveness and migratory ability in lung cancer cells after IDO1 silencing [55]. The same authors discovered that IDO1 presence conditions sufficient vessel density and the progression of vessel mimicry, which in short is unnecessary for the proper angiogenesis process. Another trial focused on lung cancer cells confirms that IDO1 activity is crucial for metastasis occurrence, and its inhibition improves patient outcomes [56]. The described data point to mutual permeating of immunological processes and cancer development, as well as indicate IDO1 as a significant factor that takes part in this mechanism.
5. TDO2, IDO2 and Their Role in Cancer Development
Two other rate-limiting enzymes are less studied than IDO1. Nevertheless, it is known that they are as well involved in maintaining oncogenesis. Their effect can partially be explained by similar to IDO1 activity toward TRP metabolism with its depletion in the local environment and the accumulation of immunosuppressive metabolites. From the pharmacological perspective, these overlapping events provide a reason for cancer cell resistance to IDO1 inhibition, as its role is taken over by the other two enzymes. However, the available data indicate that the expression level of IDO1, IDO2 and TDO2 differs between cancer types and that the occurrence of each enzyme alone can be an independent prognosis factor. This knowledge suggests that, at least in part, TDO2 or IDO2 exert a tumor-promoting role through different mechanisms than those described in IDO1 activity. In metastatic uveal melanoma, TDO2 but not IDO1 was found to be expressed in cancer tissue, in a constitutive manner [57]. In the same cell line, TNF-∝ (tumor necrosis factor-alfa) was pointed as a factor, which upregulates TDO2 expression, but has no impact on the IDO1 level. Additionally, in the case of triple-negative breast cancer, TDO2 seems to play a major role in disease progression, surpassing the importance of IDO1 [58]. Hsu et al. showed that TDO2 is expressed in lung cancer-associated fibroblasts which after knockdown disturb DCs differentiation and response from Th2 [59]. The role of the latter in cancer progression is based mainly on interleukin secretion. Th2 in a tumor microenvironment is a great source of IL-4, IL-5 and IL-13. Both IL-4 and IL-13, when in excess, have been connected with more aggressive cancers, enhanced metastasis, proliferation, and tumor growth. Detailed studies point to multiple mechanisms which are regulated by those interleukins. Well-known tumor-promoting signaling pathways, such as ERK1/2, Akt, mTOR, and STAT6 are among those induced by the mentioned factors. Moreover, it has been proved that IL-4 induces an expression of antiapoptotic proteins, such as Bax, BCL-xl, xFLIP, and contributes to sustained cancer growth through the induction of expression of glucose transporter—GLUT1 [60][61][62][63][64][65]. The aspect which cannot be omitted in the context of Th2 derived interleukins in cancer development is their role in macrophage polarization. Both IL-4 and IL-13 are involved in the differentiation of macrophages into pro-tumorigenic M2 [66], which are classified as tumor-associated macrophages (TAMs) [67]. A high presence of TAMs in the tumor microenvironment is associated with a poor prognosis in different cancer types as NSCLC, pancreatic carcinoma, ovarian cancer or gastric cancer [68][69][70][71]. Considering their involvement in cancerogenesis, it is worth mentioning, that they play a role at each stage of the ongoing process [72]. By the secretion of inflammation-promoting factors, i.e., TNF, IFN-g, ROS (reactive oxygen species) contribute to the establishment of the mutagenic microenvironment and facilitate the development of damaged cells [73]. In human glioma, ovarian cancer and clear cell renal carcinoma M2 have been reported to activate STAT3 signaling with enhanced proliferation and sustained survival of tumor cells [74][75]. As a source of proangiogenic factors, such as VEGF-A, and VEGF-C M2 contribute to angio- and lymphangiogenesis, respectively [76]. Their presence in the tumor microenvironment is connected with changes in the expression of EMT markers, such as E-cadherin, β-catenin, vimentin, and snail [77], which points to their involvement in aggressive phenotype development. TAMs enable metastasis formation and increase tumor cell invasiveness, via upregulation of metalloproteinases expression, which was reported both in vivo and in patient samples [78][79][80]. Noteworthy is the fact, that M2 can secrete exosomes, which contain specific miRNA or molecules such as ApoE and integrins, that in the next steps activate migration-inducing signaling pathways [81][82][83]. Moreover, the presence of M2 in a tumor microenvironment is considered as one of the factors responsible for the occurrence of chemoresistance [84][85] and its targeting could improve the efficacy of treatment [86]. The role of TDO2 in tumor development is not restricted only to the modulation of immune cell activity. It has been shown, that this enzyme is involved in enhancing the survival of circulating tumor cells (CTCs), through the participation in the development of resistance to anoikis (a form of programmed cell death) [87]. Due to AhR involvement in this process, the whole pathway is described below.
The last known rate-limiting enzyme involved in TRP metabolism is IDO2. Its role in tumorigenesis is significantly less examined than IDO1, thus a lot of questions about its involvement in cancerogenesis remain. Nevertheless, an important contribution of both IDO1 and IDO2 in cancer development, not mentioned before, is their involvement in NAD+ production. This dinucleotide as a final product of TRP metabolism is used as a source of energy for maintaining cells’ functions and viability. In a tumor microenvironment, an increased level of NAD+ has been shown to exert an immunosuppressive effect by the inhibition of T cell survival, proliferation and cytotoxic activity [88]. Additionally, its further metabolism takes part in enhancing immune evasion by promoting PD-L1 expression in tumor cells [89]. Its upraised level is connected with a poor prognosis and decreased overall survival in the number of solid tumors, i.e., non–small cell lung cancer, renal cell cancer, ovarian cancer, gastric cancers [90]. This ligand works through the PD-1 receptor expressed on different cell types, including activated Tcells. After interaction, the signaling from TCR attenuates [91], which is described above in the context of maintained tumor development. Additionally, recent reports suggest that PD-L1 has a direct impact on cancer cells, therefore it intensifies tumorigenesis not only via the modulation of the immune response. The correlation between an elevated level of PD-L1 and enhanced EMT was reported in a few cancer types, i.e., head and neck squamous cell carcinoma, lung adenocarcinoma, gastric cancer [92][93][94]. Wang et al. showed that in renal cancer cells PD-L1 overexpression induces the expression of SREBP-1c, a factor involved in cell lipogenesis. The authors connected this event with EMT induction and intensified cancer cell migration [95]. Other immune-independent effects caused by PD-L1 include the activation of mTORC1 and Ras/ERK and signaling cascades, with the following sustained growth of melanoma and ovarian cancer cells and enhanced EMT in glioblastoma multiforme, respectively [96][97]. Moreover, Mandarano et al. showed a correlation between upregulated IDO2 and PD-L1 levels, which indicates the existence of a link between IDO2 activity and enhanced cancerogenesis in a PD-L1-dependent manner [98]. Besides affecting PD-L1 expression, NAD+ contributes to stem cell proliferation and pluripotency [99]. However, it still needs to be confirmed if this nucleotide plays a role in maintaining the viability of cancer stem cells. There still exists a high need to define other mechanisms of IDO2 via which it contributes to cancerogenesis. However, reports showing an upraised level of this enzyme in human cancer tissues suggest that it is an important factor, which cancer cells use to maintain their survival in a host organism.
References
- Kunimasa, K.; Goto, T. Immunosurveillance and Immunoediting of Lung Cancer: Current Perspectives and Challenges. Int. J. Mol. Sci. 2020, 21, 597.
- Lussier, D.M.; Schreiber, R.D. Cancer Immunosurveillance: Immunoediting. In Immunity to Pathogens and Tumors; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 4, pp. 396–405.
- Teng, M.W.; Galon, J.; Fridman, W.H.; Smyth, M.J. From mice to humans: Developments in cancer immunoediting. J. Clin. Investig. 2015, 125, 3338–3346.
- Johnson, R.; Wen, T.; Dong, H. Bidirectional signals of PD-L1 in T cells that fraternize with cancer cells. Nat. Immunol. 2020, 21, 365–366.
- Hermanowicz, J.; Sieklucka, B.; Nosek, K.; Pawlak, D. Intracellular mechanisms of tumor cells’ immunoresistance. Acta Biochim. Pol. 2020, 67, 143–148.
- Beatty, G.L.; Gladney, W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692.
- Murray, P.J. Amino acid auxotrophy as a system of immunological control nodes. Nat. Immunol. 2016, 17, 132–139.
- Sun, B.; Hyun, H.; Li, L.T.; Wang, A.Z. Harnessing nanomedicine to overcome the immunosuppressive tumor microenvironment. Acta Pharmacol. Sin. 2020, 41, 970–985.
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724.
- Zhai, L.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.; Wainwright, D.A. IDO1 in cancer: A Gemini of immune checkpoints. Cell. Mol. Immunol. 2018, 15, 447–457.
- Venkateswaran, N.; Lafita-Navarro, M.C.; Hao, Y.H.; Kilgore, J.A.; Perez-Castro, L.; Braverman, J.; Borenstein-Auerbach, N.; Kim, M.; Lesner, N.P.; Mishra, P.; et al. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes Dev. 2019, 33, 1236–1251.
- Balachandran, V.P.; Cavnar, M.J.; Zeng, S.; Bamboat, Z.M.; Ocuin, L.M.; Obaid, H.; Sorenson, E.C.; Popow, R.; Ariyan, C.; Rossi, F.; et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 2011, 17, 1094–1100.
- Ahmadzada, T.; Lee, K.; Clarke, C.; Cooper, W.A.; Linton, A.; McCaughan, B.; Asher, R.; Clarke, S.; Reid, G.; Kao, S. High BIN1 expression has a favorable prognosis in malignant pleural mesothelioma and is associated with tumor infiltrating lymphocytes. Lung Cancer 2019, 130, 35–41.
- Ren, S.; Correia, M.A. Heme: A regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch. Biochem. Biophys. 2000, 377, 195–203.
- Bender, D.A.; Laing, A.E.; Vale, J.A.; Papadaki, L.; Pugh, M. The effects of oestrogen administration on tryptophan metabolism in rats and in menopausal women receiving hormone replacement therapy. Biochem. Pharmacol. 1983, 32, 843–848.
- Oxenkrug, G.F. Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: The serotonin hypothesis revisited 40 years later. Isr. J. Psychiatry Relat. Sci. 2010, 47, 56–63.
- Poulain-Godefroy, O.; Eury, E.; Leloire, A.; Hennart, B.; Guillemin, G.J.; Allorge, D.; Froguel, P. Induction of TDO2 and IDO2 in Liver by High-Fat Feeding in Mice: Discrepancies with Human Obesity. Int. J. Tryptophan Res. 2013, 6, 29–37.
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2017, 53, 10–27.
- Opitz, C.A.; Somarribas Patterson, L.F.; Mohapatra, S.R.; Dewi, D.L.; Sadik, A.; Platten, M.; Trump, S. The therapeutic potential of targeting tryptophan catabolism in cancer. Br. J. Cancer 2020, 122, 30–44.
- Suciu-Foca, N.; Cortesini, R. Central role of ILT3 in the T suppressor cell cascade. Cell. Immunol. 2007, 248, 59–67.
- Brenk, M.; Scheler, M.; Koch, S.; Neumann, J.; Takikawa, O.; Häcker, G.; Bieber, T.; von Bubnoff, D. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J. Immunol. 2009, 183, 145–154.
- Manavalan, J.S.; Rossi, P.C.; Vlad, G.; Piazza, F.; Yarilina, A.; Cortesini, R.; Mancini, D.; Suciu-Foca, N. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl. Immunol. 2003, 11, 245–258.
- Zhang, Y.; Lu, N.; Xue, Y.; Zhang, M.; Li, Y.; Si, Y.; Biao, X.; Jia, Y.; Wang, Y. Expression of immunoglobulin-like transcript (ILT)2 and ILT3 in human gastric cancer and its clinical significance. Mol. Med. Rep. 2012, 5, 910–916.
- Liu, J.; Wang, L.; Gao, W.; Li, L.; Cui, X.; Yang, H.; Lin, W.; Dang, Q.; Zhang, N.; Sun, Y. Inhibitory receptor immunoglobulin-like transcript 4 was highly expressed in primary ductal and lobular breast cancer and significantly correlated with IL-10. Diagn. Pathol. 2014, 9, 1–8.
- Li, J.; Gao, A.; Zhang, F.; Wang, S.; Wang, J.; Wang, J.; Han, S.; Yang, Z.; Chen, X.; Fang, Y.; et al. ILT3 promotes tumor cell motility and angiogenesis in non-small cell lung cancer. Cancer Lett. 2021, 501, 263–276.
- Liu, J.; Lu, C.X.; Zhang, F.; Lv, W.; Liu, C. Expression of ILT3 predicts poor prognosis and is inversely associated with infiltration of CD45RO+ T cells in patients with colorectal cancer. Pathol. Res. Pract. 2018, 214, 1621–1625.
- García, M.; Palma, M.B.; Verine, J.; Miriuka, S.; Inda, A.M.; Errecalde, A.L.; Desgrandchamps, F.; Carosella, E.D.; Tronik-Le Roux, D. The immune-checkpoint HLA-G/ILT4 is involved in the regulation of VEGF expression in clear cell renal cell carcinoma. BMC Cancer 2020, 20, 1–11.
- Courtney, A.H.; Lo, W.L.; Weiss, A. TCR Signaling: Mechanisms of Initiation and Propagation. Trends Biochem. Sci. 2018, 43, 108–123.
- Colligan, S.H.; Tzetzo, S.L.; Abrams, S.I. Myeloid-driven mechanisms as barriers to antitumor CD8+ T cell activity. Mol. Immunol. 2020, 118, 165–173.
- Dar, A.A.; Bhat, S.A.; Gogoi, D.; Gokhale, A.; Chiplunkar, S.V. Inhibition of Notch signalling has ability to alter the proximal and distal TCR signalling events in human CD3+ αβ T-cells. Mol. Immunol. 2017, 92, 116–124.
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367.
- Xu, X.; Tan, Y.; Qian, Y.; Xue, W.; Wang, Y.; Du, J.; Jin, L.; Ding, W. Clinicopathologic and prognostic significance of tumor-infiltrating CD8+ T cells in patients with hepatocellular carcinoma: A meta-analysis. Medicine 2019, 98, e13923.
- Han, S.; Zhang, C.; Li, Q.; Dong, J.; Liu, Y.; Huang, Y.; Jiang, T.; Wu, A. Tumour-infiltrating CD4+ and CD8+ lymphocytes as predictors of clinical outcome in glioma. Br. J. Cancer 2014, 110, 2560–2568.
- Dar, A.A.; Pradhan, T.N.; Kulkarni, D.P.; Shah, S.U.; Rao, K.V.; Chaukar, D.A.; D’Cruz, A.K.; Chiplunkar, S.V. Extracellular 2’5’-oligoadenylate synthetase 2 mediates T-cell receptor CD3-ζ chain down-regulation via caspase-3 activation in oral cancer. Immunology 2016, 147, 251–264.
- Frydecka, I.; Kaczmarek, P.; Boćko, D.; Kosmaczewska, A.; Morilla, R.; Catovsky, D. Expression of signal-transducing zeta chain in peripheral blood T cells and natural killer cells in patients with Hodgkin’s disease in different phases of the disease. Leuk. Lymphoma 1999, 35, 545–554.
- Kim, H.R.; Park, J.S.; Fatima, Y.; Kausar, M.; Park, J.H.; Jun, C.D. Potentiating the Antitumor Activity of Cytotoxic T Cells via the Transmembrane Domain of IGSF4 That Increases TCR Avidity. Front. Immunol. 2021, 11, 3667.
- Bono, M.R.; Fernández, D.; Flores-Santibáñez, F.; Rosemblatt, M.; Sauma, D. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 2015, 589, 3454–3460.
- Hajizadeh, F.; Masjedi, A.; Asl, S.H.; Kiani, F.K.; Peydaveisi, M.; Ghalamfarsa, G.; Jadidi-Niaragh, F.; Sevbitov, A. Adenosine and adenosine receptors in colorectal cancer. Int. Immunopharmacol. 2020, 87, 106853.
- Shi, L.; Wu, Z.; Miao, J.; Du, S.; Ai, S.; Xu, E.; Feng, M.; Song, J.; Guan, W. Adenosine interaction with adenosine receptor A2a promotes gastric cancer metastasis by enhancing PI3K-AKT-mTOR signaling. Mol. Biol. Cell 2019, 30, 2527–2534.
- Yan, A.; Joachims, M.L.; Thompson, L.F.; Miller, A.D.; Canoll, P.D.; Bynoe, M.S. CD73 Promotes Glioblastoma Pathogenesis and Enhances Its Chemoresistance via A2B Adenosine Receptor Signaling. J. Neurosci. 2019, 39, 4387–4402.
- Janssen, L.; Ramsay, E.E.; Logsdon, C.D.; Overwijk, W.W. The immune system in cancer metastasis: Friend or foe? J. Immunother. Cancer 2017, 5, 1–14.
- Najafi, M.; Farhood, B.; Mortezaee, K. Contribution of regulatory T cells to cancer: A review. J. Cell. Physiol. 2019, 234, 7983–7993.
- Zoso, A.; Mazza, E.M.; Bicciato, S.; Mandruzzato, S.; Bronte, V.; Serafini, P.; Inverardi, L. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur. J. Immunol. 2014, 44, 3307–3319.
- Shen, Z.; Zhou, S.; Wang, Y.; Li, R.L.; Zhong, C.; Liang, C.; Sun, Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 1585–1595.
- Li, Y.Q.; Liu, F.F.; Zhang, X.M.; Guo, X.J.; Ren, M.J.; Fu, L. Tumor secretion of CCL22 activates intratumoral Treg infiltration and is independent prognostic predictor of breast cancer. PLoS ONE 2013, 8, e76379.
- Liotta, F.; Gacci, M.; Frosali, F.; Querci, V.; Vittori, G.; Lapini, A.; Santarlasci, V.; Serni, S.; Cosmi, L.; Maggi, L.; et al. Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int. 2011, 107, 1500–1506.
- Wang, D.; Yang, L.; Yu, W.; Wu, Q.; Lian, J.; Li, F.; Liu, S.; Li, A.; He, Z.; Liu, J.; et al. Colorectal cancer cell-derived CCL20 recruits regulatory T cells to promote chemoresistance via FOXO1/CEBPB/NF-κB signaling. J. Immunother. Cancer 2019, 7, 1–15.
- Velaei, K.; Samadi, N.; Barazvan, B.; Rad, J.S. Tumor microenvironment-mediated chemoresistance in breast cancer. Breast 2016, 30, 92–100.
- Erdman, S.E.; Rao, V.P.; Olipitz, W.; Taylor, C.L.; Jackson, E.A.; Levkovich, T.; Lee, C.W.; Horwitz, B.H.; Fox, J.G.; Ge, Z.; et al. Unifying roles for regulatory T cells and inflammation in cancer. Int. J. Cancer 2010, 126, 1651–1665.
- Whiteside, T.L. What are regulatory T cells (Treg) regulating in cancer and why? Semin. Cancer Biol. 2012, 22, 327–334.
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543.
- Jordanova, E.S.; Gorter, A.; Ayachi, O.; Prins, F.; Durrant, L.G.; Kenter, G.G.; van der Burg, S.H.; Fleuren, G.J. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: Which variable determines survival of cervical cancer patients? Clin. Cancer Res. 2008, 14, 2028–2035.
- Arias, D.A.A.; Kim, H.J.; Zhou, P.; Holderried, T.A.; Wang, X.; Dranoff, G.; Cantor, H. Disruption of CD8+ Treg activity results in expansion of T follicular helper cells and enhanced antitumor immunity. Cancer Immunol. Res. 2014, 2, 207–216.
- Zhang, W.; Mao, S.; Shi, D.; Zhang, J.; Zhang, Z.; Guo, Y.; Wu, Y.; Wang, R.; Wang, L.; Huang, Y.; et al. MicroRNA-153 Decreases Tryptophan Catabolism and Inhibits Angiogenesis in Bladder Cancer by Targeting Indoleamine 2,3-Dioxygenase 1. Front. Oncol. 2019, 9, 619.
- Pan, J.; Yuan, K.; Peng, S.; Huang, Y.; Zhang, Y.; Hu, Y.; Feng, Y.; Shi, Y.; Liu, Y.; Wang, H.; et al. Gene silencing of indoleamine 2,3-dioxygenase hinders tumor growth through angiogenesis inhibition. Int. J. Oncol. 2017, 50, 2136–2144.
- Mondal, A.; Smith, C.; DuHadaway, J.B.; Sutanto-Ward, E.; Prendergast, G.C.; Bravo-Nuevo, A.; Muller, A.J. IDO1 is an Integral Mediator of Inflammatory Neovascularization. EBioMedicine 2016, 14, 74–82.
- Terai, M.; Londin, E.; Rochani, A.; Link, E.; Lam, B.; Kaushal, G.; Bhushan, A.; Orloff, M.; Sato, T. Expression of Tryptophan 2,3-Dioxygenase in Metastatic Uveal Melanoma. Cancers 2020, 12, 405.
- Liu, Q.; Zhai, J.; Kong, X.; Wang, X.; Wang, Z.; Fang, Y.; Wang, J. Comprehensive Analysis of the Expressionand Prognosis for TDO2 in Breast Cancer. Mol. Ther. Oncolytics 2020, 17, 153–168.
- Hsu, Y.L.; Hung, J.Y.; Chiang, S.Y.; Jian, S.F.; Wu, C.Y.; Lin, Y.S.; Tsai, Y.M.; Chou, S.H.; Tsai, M.J.; Kuo, P.L. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget 2016, 7, 27584–27598.
- Venmar, K.T.; Carter, K.J.; Hwang, D.G.; Dozier, E.A.; Fingleton, B. IL4 receptor ILR4α regulates metastatic colonization by mammary tumors through multiple signaling pathways. Cancer Res. 2014, 74, 4329–4340.
- Jiang, L.; Cheng, Q.; Zhang, B.; Zhang, M. IL-13 induces the expression of 11βHSD2 in IL-13Rα2 dependent manner and promotes the malignancy of colorectal cancer. Am. J. Transl. Res. 2016, 8, 1064–1072.
- Suzuki, A.; Leland, P.; Joshi, B.H.; Puri, R.K. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine 2015, 75, 79–88.
- Guruprasath, P.; Kim, J.; Gunassekaran, G.R.; Chi, L.; Kim, S.; Park, R.W.; Kim, S.H.; Baek, M.C.; Bae, S.M.; Kim, S.Y.; et al. Interleukin-4 receptor-targeted delivery of Bcl-xL siRNA sensitizes tumors to chemotherapy and inhibits tumor growth. Biomaterials 2017, 142, 101–111.
- Hallett, M.A.; Venmar, K.T.; Fingleton, B. Cytokine stimulation of epithelial cancer cells: The similar and divergent functions of IL-4 and IL-13. Cancer Res. 2012, 72, 6338–6343.
- Venmar, K.T.; Kimmel, D.W.; Cliffel, D.E.; Fingleton, B. IL4 receptor α mediates enhanced glucose and glutamine metabolism to support breast cancer growth. Biochim. Biophys. Acta 2015, 1853, 1219–1228.
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835.
- Ma, X.; Wu, D.; Zhou, S.; Wan, F.; Liu, H.; Xu, X.; Xu, X.; Zhao, Y.; Tang, M. The pancreatic cancer secreted REG4 promotes macrophage polarization to M2 through EGFR/AKT/CREB pathway. Oncol. Rep. 2016, 35, 189–196.
- Cao, L.; Che, X.; Qiu, X.; Li, Z.; Yang, B.; Wang, S.; Hou, K.; Fan, Y.; Qu, X.; Liu, Y. M2 macrophage infiltration into tumor islets leads to poor prognosis in non-small-cell lung cancer. Cancer Manag. Res. 2019, 11, 6125–6138.
- Hu, H.; Hang, J.J.; Han, T.; Zhuo, M.; Jiao, F.; Wang, L.W. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol. 2016, 37, 8657–8664.
- Yuan, X.; Zhang, J.; Li, D.; Mao, Y.; Mo, F.; Du, W.; Ma, X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2017, 147, 181–187.
- Räihä, M.R.; Puolakkainen, P.A. Tumor-associated macrophages (TAMs) as biomarkers for gastric cancer: A review. Chronic Dis. Transl. Med. 2018, 4, 156–163.
- Salmaninejad, A.; Valilou, S.F.; Soltani, A.; Ahmadi, S.; Abarghan, Y.J.; Rosengren, R.J.; Sahebkar, A. Tumor-associated macrophages: Role in cancer development and therapeutic implications. Cell. Oncol. 2019, 42, 591–608.
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020, 353, 104119.
- Komohara, Y.; Horlad, H.; Ohnishi, K.; Fujiwara, Y.; Bai, B.; Nakagawa, T.; Suzu, S.; Nakamura, H.; Kuratsu, J.; Takeya, M. Importance of direct macrophage-tumor cell interaction on progression of human glioma. Cancer Sci. 2012, 103, 2165–2172.
- Komohara, Y.; Hasita, H.; Ohnishi, K.; Fujiwara, Y.; Suzu, S.; Eto, M.; Takeya, M. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011, 102, 1424–1431.
- Aras, S.; Zaidi, M.R. TAMeless traitors: Macrophages in cancer progression and metastasis. Br. J. Cancer 2017, 117, 1583–1591.
- Hu, Y.; He, M.Y.; Zhu, L.F.; Yang, C.C.; Zhou, M.L.; Wang, Q.; Zhang, W.; Zheng, Y.Y.; Wang, D.M.; Xu, Z.Q.; et al. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 1–19.
- Vinnakota, K.; Zhang, Y.; Selvanesan, B.C.; Topi, G.; Salim, T.; Sand-Dejmek, J.; Jönsson, G.; Sjölander, A. M2-like macrophages induce colon cancer cell invasion via matrix metalloproteinases. J. Cell. Physiol. 2017, 232, 3468–3480.
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690.
- Fields, G.B. Mechanisms of Action of Novel Drugs Targeting Angiogenesis-Promoting Matrix Metalloproteinases. Front. Immunol. 2019, 10, 1278.
- Lan, J.; Sun, L.; Xu, F.; Liu, L.; Hu, F.; Song, D.; Hou, Z.; Wu, W.; Luo, X.; Wang, J.; et al. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res. 2019, 79, 146–158.
- Zheng, P.; Luo, Q.; Wang, W.; Li, J.; Wang, T.; Wang, P.; Chen, L.; Zhang, P.; Chen, H.; Liu, Y.; et al. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018, 9, 1–14.
- Wu, J.; Gao, W.; Tang, Q.; Yu, Y.; You, W.; Wu, Z.; Fan, Y.; Zhang, L.; Wu, C.; Han, G.; et al. M2 macrophage-derived exosomes facilitate hepatocarcinoma metastasis by transferring αM β2 integrin to tumor cells. Hepatology 2020.
- Ireland, L.V.; Mielgo, A. Macrophages and Fibroblasts, Key Players in Cancer Chemoresistance. Front. Cell Dev. Biol. 2018, 6, 131.
- An, Y.; Yang, Q. MiR-21 modulates the polarization of macrophages and increases the effects of M2 macrophages on promoting the chemoresistance of ovarian cancer. Life Sci. 2020, 242, 117162.
- Zhao, P.; Yin, W.; Wu, A.; Tang, Y.; Wang, J.; Pan, Z.; Lin, T.; Zhang, M.; Chen, B.; Duan, Y.; et al. Dual-targeting to Cancer Cells and M2 macrophages via Biomimetic Delivery of Mannosylated Albumine Nanoparticles for Drug-Resistant Cancer Therapy. Adv. Funct. Mater. 2017, 27, 1700403.
- D’Amato, N.C.; Rogers, T.J.; Gordon, M.A.; Greene, L.I.; Cochrane, D.R.; Spoelstra, N.S.; Nemkov, T.G.; D’Alessandro, A.; Hansen, K.C.; Richer, J.K. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015, 75, 4651–4664.
- Mottahedeh, J.; Haffner, M.C.; Grogan, T.R.; Hashimoto, T.; Crowell, P.D.; Beltran, H.; Sboner, A.; Bareja, R.; Esopi, D.; Isaacs, W.B.; et al. CD38 is methylated in prostate cancer and regulates extracellular NAD. Cancer Metab. 2018, 6, 1–17.
- Lv, H.; Lv, G.; Chen, C.; Zong, Q.; Jiang, G.; Ye, D.; Cui, X.; He, Y.; Xiang, W.; Han, Q.; et al. NAD+ Metabolism Maintains Inducible PD-L1 Expression to Drive Tumor Immune Evasion. Cell Metab. 2021, 33, 110–127.e5.
- Mandai, M.; Hamanishi, J.; Abiko, K.; Matsumura, N.; Baba, T.; Konishi, I. Dual Faces of IFNγ in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin. Cancer Res. 2016, 22, 2329–2334.
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671–97682.
- Ock, C.Y.; Kim, S.; Keam, B.; Kim, M.; Kim, T.M.; Kim, J.H.; Jeon, Y.K.; Lee, J.S.; Kwon, S.K.; Hah, J.H.; et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 15901–15914.
- Kim, S.; Koh, J.; Kim, M.Y.; Kwon, D.; Go, H.; Kim, Y.A.; Jeon, Y.K.; Chung, D.H. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum. Pathol. 2016, 58, 7–14.
- Chen, L.; Xiong, Y.; Li, J.; Zheng, X.; Zhou, Q.; Turner, A.; Wu, C.; Lu, B.; Jiang, J. PD-L1 Expression Promotes Epithelial to Mesenchymal Transition in Human Esophageal Cancer. Cell. Physiol. Biochem. 2017, 42, 2267–2280.
- Wang, Y.; Wang, H.; Zhao, Q.; Xia, Y.; Hu, X.; Guo, J. PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med. Oncol. 2015, 32, 1–7.
- Clark, C.A.; Gupta, H.B.; Sareddy, G.; Pandeswara, S.; Lao, S.; Yuan, B.; Drerup, J.M.; Padron, A.; Conejo-Garcia, J.; Murthy, K.; et al. Tumor-Intrinsic PD-L1 Signals Regulate Cell Growth, Pathogenesis, and Autophagy in Ovarian Cancer and Melanoma. Cancer Res. 2016, 76, 6964–6974.
- Qiu, X.Y.; Hu, D.X.; Chen, W.Q.; Chen, R.Q.; Qian, S.R.; Li, C.Y.; Li, Y.J.; Xiong, X.X.; Liu, D.; Pan, F.; et al. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochimica et biophysica acta. Mol. Basis Dis. 2018, 1864, 1754–1769.
- Mandarano, M.; Bellezza, G.; Belladonna, M.L.; Vannucci, J.; Gili, A.; Ferri, I.; Lupi, C.; Ludovini, V.; Falabella, G.; Metro, G.; et al. Indoleamine 2,3-Dioxygenase 2 Immunohistochemical Expression in Resected Human Non-small Cell Lung Cancer: A Potential New Prognostic Tool. Front. Immunol. 2020, 11, 839.
- Navas, L.E.; Carnero, A. NAD+ metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 2021, 6, 1–20.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
819
Revisions:
2 times
(View History)
Update Date:
16 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




