
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | giuseppe De Mastro | + 3517 word(s) | 3517 | 2021-04-26 09:56:31 | | | |
| 2 | Vivi Li | Meta information modification | 3517 | 2021-04-27 03:04:22 | | |
Video Upload Options
In all farming systems, weeds are the most expensive pest to manage, accounting for 30% of potential losses. In organic farming, the problem may be further amplified by restrictions on herbicides, thus making weeds the main problem faced by organic farmers in the field. In this sense, much research is focusing on the allelopathic potential of plants as an ecological weed control tool. Many plant species can release allelopathic compounds with high phytotoxicity that can be used in weed control. Species belonging to the Lamiaceae family have been studied widely for this purpose, and their essential oils (EOs) appear to be promising bioherbicides. However, there are still many challenges for their development.
1. Introduction
The emerging worldwide need to find alternatives to synthetic herbicides for sustainable weed control has prompted considerable interest in exploiting the natural herbicidal potential in plants [1]. Bioherbicide sources are sought out by both conventional and organic farming systems: the former wish to identify new sites of action to cope with weed resistance, the latter seek potent alternatives to synthetic herbicides that can be integrated in an overall management approach [2]. In this context, weed control research has recently focused on extracts from allelopathic species. These are species that can release secondary metabolites able to interfere with the growth and functions of surrounding plants [3].
A well-established group of allelopathic plants is that of the Lamiaceae family. They are known to contain high concentrations of volatile allelochemicals, which are responsible for their aroma, and are reported to give the species a competitive advantage in their natural habitats [4]. In this context, extracts of different Lamiaceae species were studied extensively and found to inhibit the germination and growth of many weed species [5][6][7][8][9][10][11][12]. Essential oils (EOs) from species such as oregano, thyme, rosemary, sage and mint are reported to be particularly strong bioherbicide candidates.
The phytotoxic effect of these species extracts, notably EOs, has mainly been linked to the presence of volatile bio-active compounds such as α-pinene, limonene, 1,8-cineole, carvacrol, camphor and thymol, which have been shown to have varying individual phytotoxicity levels [4][11][12][13][14][15]. Some phenolic compounds present in the EOs were also reported to be involved in allelopathic interactions and were even used to develop commercial bioherbicides. The mechanisms by which these allelochemicals can affect weeds was not discussed in detail. Only a few individual compounds were studied [16][17][18], in addition to the mechanism behind some naturally occurring allelopathic interactions [16][19][20][21].
Although there are numerous studies reporting on the successful use of EOs in weed control, to date there are still many constraints limiting their practical application in commercial bioherbicides. For instance, the role of the EO composition is still not clearly described. The mechanisms of action and the observed selectivity are also very poorly understood, limiting their rational implementation. Moreover, studies concerning the possible side effects of these EOs on beneficial soil microorganisms are still lacking.
2. The Use of Plant-Based Bioherbicides
2.1. Bioherbicidal Potential in Plants
The interest in exploiting the natural herbicidal potential of plants stems from a worldwide need to find new sustainable weed control strategies [1].
As plants are the richest source of active organic compounds on Earth, the bioherbicidal potential of a long list of plant species has been explored [22]. A Scopus literature search using the keywords « Bioherbicides AND plant extracts » and « Bioherbicides AND Essential oils » found 130 articles (excluding review articles) on the bioherbicidal potential of species from 38 different families (Figure 1).
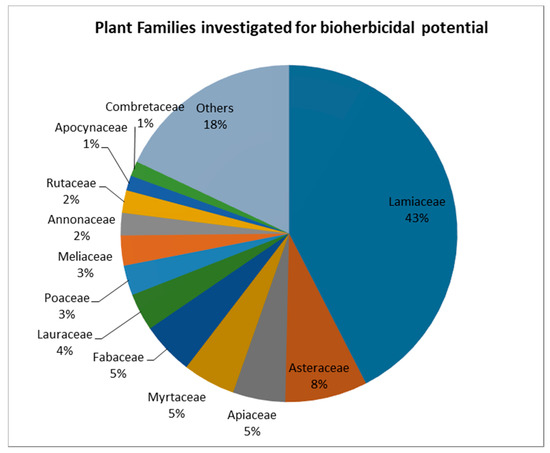
Figure 1. Plant families studied for their bioherbicidal potential. (Source: elaborated from a search on Scopus, 2019).
Plant species can be considered for investigations due to their known composition in terms of biologically active compounds, or an observed allelopathic effect in their natural environment. Allelopathy is a characteristic of many plant species, and can be defined as a form of interaction between plants through chemical inhibitors released from living or decaying tissues [3]. Evidence that some plants are able to inhibit the growth of other plants in their surroundings has long been known and reported, and studies have linked these interactions to the presence of compounds named “allelochemicals” [4][21][23]. This can justify the high interest in the Lamiaceae family species—accounting for 43% of the total studied species from the papers covered by the search—which are known to possess high concentrations of volatile allelochemicals. In this context, much effort has been made to extract allelochemicals from plants and test their bioherbicidal activity in bioassays; many were successful (Table 1). Other studies evaluated the activity of single compounds isolated from plants, such as flavonoids, alkaloids and terpenoids. These different classes of secondary metabolites have different importance in term of bioherbicidal activity. In extracts from the same species, they can occur in different proportions, depending on the type of extract and the extraction method [24].
Table 1. Examples of frequently tested families for bioherbicidal activity.
| Species | Family | Bio Herbicidal Effect | Reference |
|---|---|---|---|
| Xanthium strumarium L. | Asteraceae | Significant inhibition of germination and growth of the noxious weed Bidens pilosa L. | [25] |
| Thymus fontanesii Boiss. et Reut. Satureja calamintha subsp. nepeta Briq. |
Lamiaceae | Wide herbicidal effect on seed germination and 3–4 leaf stage of Sinapis arvensis L, Avena fatua L., Sonchus oleraceus L., Xanthium strumarium L., Cyperus rotundus L. | [26] |
| Ulex europaeus L. Cytisus scoparius L. |
Fabaceae | Pure volatile organic compounds extracted caused irreversible phytotoxicity for Digitaria sanguinalis L. | [27] |
| Trachyspermum copticum L. | Apiaceae | Germination and shoot/root length of Zea mays L. and Lepidium sativum L. significantly reduced by all concentrations of EO and methanol extract. | [28] |
| Eucalyptus citriodora Hook | Myrtaceae | Parthenium hysterophorus L.: Germination completely inhibited. Chlorophyll content and respiratory activity decreased for 4-week-old plants. |
[29] |
2.2. Types of Active Compounds and Plant Extracts Tested as Bioherbicides
2.2.1. Active Compounds with Bioherbicidal Potential
The term active compounds usually refers to secondary metabolites occurring in plants, known for having diverse biological activities. These are the compounds with no relevance to vital functions (like respiration, photosynthesis and reproduction), but involved in interactions between plants and their surrounding environment, notably as part of their mechanism of defense against stress [30][31]. Secondary metabolites in plants have been classified differently by different authors; a recent review by Yasri et al., [32] defined four main groups: terpenoids, phenolics, sulphur-containing secondary metabolites and nitrogen-containing secondary metabolites. Not all of these groups of secondary metabolites were found to be implicated in allelopathic interactions or showed a bioherbicidal potential. Although some authors included amino acids and proteins among the phytotoxic compounds, terpenoids and phenolics were the ones most frequently studied [4]. Only these two groups will therefore be considered in detail in this paragraph.
-
(a)—Terpenoids
The terpenoid group is present in the majority of secondary metabolite classifications and is reported to be very important in allelopathic interactions [4][21][33]. The compounds of this group, sometimes referred to as volatile allelochemicals, can be divided into monoterpenes, sesquiterpenes, diterpene, triterpenes and polyterpenes [32]. Monoterpenes are the major constituents of essential oils and have been shown to inhibit seed germination and seedling growth [14]. They are the most frequently described secondary metabolites for bioherbicidal activity [4][14][32][33][34][35][36]. Some monoterpene-based commercial herbicides have been developed such as cinmethylin, which is a derivative of 1,4-cineole [14].
Most authors considered that ketone-containing compounds such as camphor and pulegone are the most toxic, followed by alcohol compounds such as cineol and citronellol, and by ether, diene and monoene compounds such as α-pinene, which are the least toxic [37]. This was confirmed by many other authors [14][37]. Considering that plant species and chemotypes have different monoterpene composition, the phytotoxicity of extracts can vary between plant materials with different percentages of effective compounds (e.g camphor). However, there is no clear evidence reported in the literature as to how the active compounds of a plant extract define its activity level. In other words, it is not clear whether the observed toxic effect of plant extracts is due to the potent phytotoxicity of a single compound or to the synergic action of many constituents.
-
(b)—Phenolics
Plant phenolics include phenolic acids, flavonoids and tannins. They are synthetized by plants as a response to ecological and physiological conditions, mainly when they are under biotic or abiotic stress [38]. Like the terpenoids, an important focus exists on the identification of phenols with bioherbicidal activity. This was attributed to the easiness of their extraction and their water solubility [37]. They are usually the main components in aqueous and organic solvents extracts, and their polarity determines the type and amount of phenols extracted. An example of a well-studied phenolic for this effect is juglone (Figure 2) produced by walnuts [39].
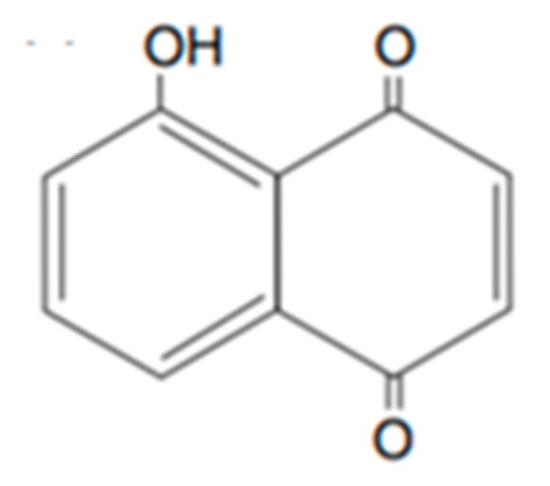
Figure 2. Representative structure of juglone.
2.2.2. Types of Plant Extracts Tested for their Bioherbicidal Activity
The extraction method is a determining factor in the recovery of active compounds from plants, considering that secondary metabolites of different groups have varying chemical properties (volatility, polarity etc.). For instance, anthocyanins, tannins, saponins and terpenoids can be recovered using water, whereas polyphenols, flavonoids, flavones and alkaloids require organic solvents [24]. Like the terpenoids, much research focuses on identifying phenols with bioherbicidal potential. This is because they are easily extracted and are soluble in water [33]. They are usually the main components in aqueous and organic solvent extracts, the polarity of which determines the type and amount of phenols extracted. An example of a well-studied phenolic with this effect is juglone (Figure 2), produced by walnuts [39]. These different extracts often show different levels of toxicity. In a study conducted to test Calamintha nepeta L. (Savi) as a source of phytotoxic compounds, solvents of varying polarity (n-hexane, chloroform, ethyl acetate and n-butanol) were used to fractionate the leaves’ methanol extract. The study defined the following hierarchical phytotoxicity: ethyl acetate > n-hexane > chloroform > n-butanol [40].
In general, three main groups of extracts can be found in the literature: essential oils (EOs), aqueous extract and organic solvent extracts.
-
Essential oils: Sometimes called volatile oils, these are natural substances that can be extracted from aromatic plants by distillation or by appropriate mechanical process without heating. EOs mainly contain compounds that can be volatilized during extraction, making this an effective means of extracting plant terpenoids in the purest form [18][41]. These, the most frequently tested extracts from aromatic plants, can cause higher phytoxicity compared to aqueous or organic solvent extracts [28][40][42].
-
Aqueous extracts: These are obtained simply by soaking in water ground dry material from plants, from which water-soluble compounds are extracted. Several phenols are water soluble and can successfully be extracted using this method. Aqueous extracts have been used to investigate the bioherbicidal potential of many plants and have been found to produce significant effects mainly at the highest tested concentrations [1][43][44].
-
Organic solvent extracts. This group consists mainly of phenols. As the type of solvent (mainly differing in polarity) affects the amount and type of phenols extracted, authors have used various solvents. Methanol, ethanol, acetone, ethyl acetate, n-hexane and chloroform are among those used most frequently [38]. The choice of solvent depends on the types of phenol present in the tested plant, and many authors have tested different ones simultaneously in order to compare the composition and phytotoxicity of the resulting extract [40][45].
2.3. Modes of Action of Plant Allelochemicals
After investigating the type of active compounds in the plants’ extracts, research has also explored the mechanisms of toxicity to weeds. The most frequently described effects are from single allelochemicals rather than whole plant extracts; the modes of action of terpenoids and phenolic acids, which are reported to be the most relevant secondary metabolites in allelopathic interactions, have been studied by many authors. However, studies on this topic are still lacking and the mechanism of only a few phytotoxic compounds has been described. This paragraph therefore focuses mainly on toxicity mechanisms reported for single allelochemicals, as well as allelopathic mechanisms observed in nature. Note that to determine the stage in which plants are most sensitive to allelochemicals, the latter were often tested in two different periods (pre-germination and post-emergence), and different features and mechanisms were analyzed accordingly.
2.3.1. Effect on Cells Division, Elongation and Structure
The size and weight of weed seedlings are the features most often measured to assess their reaction to the application of allelopathic compounds. The application of plant extracts usually results in a significant decrease of these parameters compared to the control. The substances undoubtedly affect the responsible physiological processes: cell division and elongation [21]. In this sense some studies have reported that some allelochemicals affect mitosis: the process was either slowed down [19], interrupted in the anaphases or hindered altogether [16][19][20][46][47]. All the cited studies measured the number of cells and their ultrastructure at specific times as indicators. Muller [16] also reported that volatile terpenes extracted from Salvia leucophylla Greene (mainly cineol and camphor), prevented the elongation of root and hypocotyl cells. Cineole is in fact the most widely described of all monoterpenes [48]. It is generally reported to strongly inhibit all stages of mitosis. The suggested mechanism can therefore result in considerable damage to weeds by reducing their growth or retarding it, which can give the crop a competitive advantage.
2.3.2. Effect on the Cells Membrane Integrity and Permeability
Cell membrane integrity is critical for cell functions and survival. Any alteration may compromise its role as a barrier, affecting permeability to nutrients or toxins or inducing the leakage of solutes [49][50]. A number of allelochemicals seem to alter plant cell membranes. Due to lipophilic nature of the cell membranes, monoterpenes can cause their destruction by increasing permeability or inhibiting enzymes [18]. Moreover, some monoterpenes are reported to induce oxidative stress; α-pinene, for example, caused lipid peroxidation when applied to young seedlings of Cassia occidentalis L., resulting in an increase in solute leakage [51]. Furthermore, some compounds produced changes to the permeability of membranes; Varona et al. [52] found that linalool caused an increase in permeability, whereas Muller et al. [16] found that permeability decreased after applying cineole and dipentene from S. leucophylla. This suggests that allelochemicals can result in important damage to weeds by acting at the membrane level.
2.3.3. Effect on Photosynthesis
There is evidence of a relationship between the visible effects on weeds and photosynthetic functions. Early studies found a correlation between the reduction in growth caused by a plant-extracted phenolic substance, “scopoletin”, and net photosynthesis in Amarantus retroflexus L. [53]. Many other studies found that a number of phenolic acids affect photosynthesis, and this was linked to changes to stomatal conductance or to plant chlorophyll contents [53][54]. Furthermore, many monoterpenes were also found to inhibit photosynthesis and chlorophyll synthesis [55]. Citronellol and 1,8-cineole, for example, showed a similar effect on the invasive weed species Ageratum conyzoides L.: its chlorophyll content decreased by 60% and 66%, respectively [18][56]. Eugenol, another monoterpene, has a similar effect: it induced photosynthetic inhibition by reducing chlorophyll content in C. occidentalis and Bidens pilosa L. [57]. These examples suggest that photosynthesis-related processes could be behind the observed damage. However, only a few of the allelochemicals were tested, and the actual cause-effect between the described processes is not yet well understood.
2.3.4. Effect on Nutrients Availability and Uptake
Because of the observed effects on the root appearance, some research has focused on whether allelochemicals inhibit nutrient uptake [21]. The uptake of phosphorous, potassium, calcium and zinc, for example, was affected either by the direct application of some phenolic acids or by growing plants in association with allelopathic species [58][59][60][61][62][63]. Moreover, some early studies found that toxic excretions from plants reduce the availability of nutrients by affecting nutrient cycling mechanisms; mineralization, for example, was suppressed by the root excretion of some natural forest vegetation due to its toxicity to the nitrification process [64]. This suggests that phytotoxic compounds from plants may affect soil microbial activity, which plays an essential role in making important nutrients like nitrogen available to plants.
All the presented modes of action suggest that allelochemicals have a strong potential as weed control tools. However, they also highlight the many challenges to their practical application. For instance, no clear selectivity can be concluded from the reported mechanisms, which means that crops may also be susceptible. Moreover, the impact on crop and soil health is also of concern if the allelochemicals have a detrimental effect on beneficial soil microbes. This, in addition to other possible challenges, will be detailed in the next paragraph.
2.4. Challenges and Perspectives to the Use of Plant Extracts as Bioherbicides
2.4.1. Challenges to the Use of Plant Extracts as Bioherbicides
-
(a)—Unclear selectivity
Although allelochemicals may affect specific functions like photosynthesis or respiration, they lack site specificity, which excludes their use as selective bioherbicides. This also means they could be phytotoxic to crops and must be managed carefully when applied. However, many studies that tested plant extracts on different weed species revealed varying degrees of sensitivity. In most cases monocots were more resistant than dicots [7][8][65]. Moreover, it was frequently reported that many crops were less affected than weeds; for instance, when applying the EO of Satureja hortensis L. and Laurus nobilis L. at low concentrations, A. retroflexus germination decreased significantly whereas tomatoes were unaffected. However, at the highest tested concentration, tomato germination also decreased, albeit at a lower rate than A. retroflexus [8]. Similar results were obtained when applying Origanum onites L. and Rosmarinus officinalis L. on Avena sterilis L., Sinapis arvensis L. and a number of wheat cultivars, where the latter were less affected [7]. This suggests that careful dosage may resolve phytotoxicity to crops. Nevertheless, studies were not able to explain this variation in sensitivity, which makes it difficult to predict and exploit. Further research is required to better understand the mechanism of action of different allelochemicals and the synergies by which they operate in plant extracts.
-
(b)—Toxicity to soil microorganisms
Organic farming relies on soil health and natural soil processes to satisfy crop nutrient needs and ensure long term fertility. In fact, one of the serious drawbacks of synthetic chemicals is their impact on soil biodiversity and their harmful effect on beneficial organisms. Plant extracts with similar effects cannot be recommended regardless of their possible effectiveness on weeds. Only a few studies have addressed this important aspect. As mentioned in the “effect on nutrients uptake and availability” paragraph, some allelochemicals may be detrimental to nitrification bacteria [64]. Moreover, many plant extracts, notably those from the Lamiaceae family, have been shown to possess antimicrobial properties [66][67][68]. Doubts may thus arise about their possible harmful effects on soil microbes. However, other studies have reported a positive impact in this respect; volatile substances from alfalfa (Medicago sativa L.), for example, induced a rapid increase in microbial respiration and fungi mycelium growth when added to the soil. Results thus suggest a possible beneficial effect on the initial colonization stage of plant residue decomposers [69]. The different findings may be ascribed to variations in the concentration of compounds in contact with microorganisms.
In summary, the soil microbial community seems to be affected by allelochemicals (either negatively or positively). Hence, when assessing the use of plant extracts as agrochemicals, care should also be taken to detect any possible negative repercussions on soil life, a crucial component of any sustainable management strategy.
-
(c)—Degradation of plant extracts in the environment
While the incorporation of allelopathic plant species biomass into the soils is constrained by the difficulty in accumulating active concentrations [21], the direct use of concentrated extracts is mainly limited by susceptibility to environmental elements. Once released in the environment, the extracts are subject to decomposition either by microorganisms or by chemical reactions. Blum [70] reported in the book chapter «Fate of phenolic allelochemicals in soils − the role of soil and rhizosphere microorganisms» that because microorganisms use phenolic acids as a source of carbon or energy, they are thus more subject to microbial transformation and utilization than to other processes (ionization, oxidation, sorption onto soil particles, fixation into the recalcitrant organic matter (e.g., polymerization)). In this chapter the author reports results from many studies suggesting that this degradation is very likely and that phenolic acids are unlikely to produce any phytotoxic effects. Moreover, Marmulla and Harder [71] report that monoterpenes such as d-limonene, α-pinene, γ-terpinene and terpinolene are readily biodegradable. They also found that different monoterpenes show different susceptibility to degradation. In addition, many allelochemicals are highly susceptible to spontaneous decomposition; abiotic photochemical processes in the atmosphere can result in lifetimes of minutes to hours, as cited by the same authors [21]. However, very little is known about their abiotic degradation in soil [67]. These aspects suggest that allelochemicals may lack the necessary persistence to be effective bioherbicides. This may be remedied by selecting critical stages of weed growth. Even a brief period of phytotoxicity could affect the competitive ability of weeds with respect to crops [67]. Another approach recently under study is the use of innovative formulations that could regulate the rate of release without compromising the desired concentration levels. For instance, experiments with rosemary EO encapsulated in a starch matrix were successful [72].
2.4.2. Perspectives for the Use of Plant Extracts as Bioherbicides
Despite the many constraints, the use of plant extracts for weed control is still considered a field with great potential. However, to address limitations, research should focus on better understanding the phenomena in terms of:
-
Linking the observed effects of extracts to the action of specific compounds and their synergies;
-
Defining the mechanisms behind the phytotoxicity to enhance it and understand the selectivity;
-
Defining the most sensitive stages of weed development to increase effectiveness and tackle the problem of the limited duration of the effect;
-
Defining innovative formulations that take into consideration the interactions of the extracts with field conditions (soil texture, microorganisms and abiotic factors such as light and temperature);
-
Defining innovative techniques for the cultivation and extraction of essential oils to guarantee the commercial feasibility of a mass production large quantity of EOs;
-
Defining formulations that allow for containing the concentrations of EOs within technical limits for an easy application on an agricultural scale.
References
- Puig, C.G.; Reigosa, M.J.; Valentão, P.; Andrade, P.B.; Pedrol, N. Unravelling the bioherbicide potential of Eucalyptus globulus Labill: Biochemistry and effects of its aqueous extract. PLoS ONE 2018, 13, e0192872.
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.-P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop. Prot. 2016, 87, 44–49.
- Zimdahl, R.L. Allelopathy. In Fundamentals of Weed Science, 5th ed.; Zimdahl, R.L., Ed.; Academic Press: Fort Collins, CO, USA, 2018; pp. 253–270.
- Ramezani, S.; Saharkhiz, M.J.; Ramezani, F.; Fotokian, M.H. Use of Essential Oils as Bioherbicides. J. Essent. Oil Bear. Plants 2008, 11, 319–327.
- Chen, F.; Peng, S.; Chen, B.-M.; Ni, G.; Liao, H. Allelopathic potential and volatile compounds of Rosmarinus officinalis L. against weeds. Allelopath. J. 2013, 32, 57–66.
- Kashkooli, A.B.; Saharkhiz, M.J. Essential Oil Compositions and Natural Herbicide Activity of Four Denaei Thyme (Thymus daenensis Celak.) Ecotypes. J. Essent. Oil Bear. Plants 2014, 17, 859–874.
- Atak, M.; Mavi, K.; Uremis, I. Bioherbicidal effects of oregano and rosemary essential oils on germination and seedling growth of bread wheat cultivars and weeds. Rom. Biotechnol. Lett. 2016, 21, 11149–11158.
- Hazrati, H.; Saharkhiz, M.J.; Moein, M.; Khoshghalb, H. Phytotoxic effects of several essential oils on two weed species and Tomato. Biocatal. Agric. Biotechnol. 2018, 13, 204–212.
- Matković, A.; Marković, T.; Vrbničanin, S.; Sarić-Krsmanović, M.; Božić, D. Chemical composition and in vitro herbicidal activity of five essential oils on Johnson grass (Sorghum halepense [L.] Pers.). Lek. Sirovine 2018, 38, 44–50.
- Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V. Potential Effects of Essential Oils Extracted from Mediterranean Aromatic Plants on Target Weeds and Soil Microorganisms. Plants 2020, 9, 1289.
- Abd-Elgawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; ElShamy, A.I. Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants 2020, 10, 36.
- Maccioni, A.; Santo, A.; Falconieri, D.; Piras, A.; Farris, E.; Maxia, A.; Bacchetta, G. Phytotoxic effects of Salvia rosmarinus essential oil on Acacia saligna seedling growth. Flora-Morphol. Distrib. Funct. Ecol. Plants 2020, 269, 151639.
- Muller, W.H.; Lorber, P.; Haley, B.; Johnson, K. Volatile Growth Inhibitors Produced by Salvia leucophylla: Effect on Oxygen Uptake by Mitochondrial Suspensions. Bull. Torrey Bot. Club 1969, 96, 89–96.
- Vaughn, S.F.; Spencer, G.F. Volatile Monoterpenes as Potential Parent Structures for New Herbicides. Weed Sci. 1993, 41, 114–119.
- Alipour, M.; Saharkhiz, M.J. Phytotoxic activity and variation in essential oil content and composition of Rosemary (Rosmarinus officinalis L.) during different phenological growth stages. Biocatal. Agric. Biotechnol. 2016, 7, 271–278.
- Muller, W.H. Volatile Materials Produced by Salvia leucophylla: Effects on Seedling Growth and Soil Bacteria. Int. J. Plant Sci. 1965, 126, 195–200.
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M. Allelopathy as a new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23.
- Grana, E.; Sanchez-Moreiras, A.M.; Reigosa, M.J. Mode of Action of Monoterpenes in Plant-Plant Interactions. Curr. Bioact. Compd. 2012, 8, 80–89.
- Cornman, I. The responses of onion and lily mitosis to coumarin and parasorbic acid. J. Exp. Biol. 1947, 23, 292–297.
- Jensen, T.E.; Welbourne, F. The Cytological Effects of Growth Inhibitors on Excised Roots of Vicia Faba and Pisum Sativum. 1962. Available online: (accessed on 22 July 2019).
- Rice, E.L. Allelopathy, 2nd ed.; Rice, E.L., Ed.; Academic Press: San Diego, CA, USA, 1984; pp. 8–344.
- Flamini, G. Natural Herbicides as a Safer and More Environmentally Friendly Approach to Weed Control: A Review of the Literature Since 2000. In Studies in Natural Products Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2012; Volume 37, pp. 353–396.
- Molisch, H. Der Einfluss einer Pflanze auf die Andere, Allelopathie. Nat. Cell Biol. 1938, 141, 493.
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436.
- El-Gawad, A.A.; ElShamy, A.; El Gendy, A.E.-N.; Gaara, A.; Assaeed, A. Volatiles Profiling, Allelopathic Activity, and Antioxidant Potentiality of Xanthium Strumarium Leaves Essential Oil from Egypt: Evidence from Chemometrics Analysis. Molecules 2019, 24, 584.
- Benchaa, S.; Hazzit, M.; Zermane, N.; Abdelkrim, H. Chemical composition and herbicidal activity of essential oils from two Labiatae species from Algeria. J. Essent. Oil Res. 2019, 31, 335–346.
- Pardo-Muras, M.; Puig, C.G.; López-Nogueira, A.; Cavaleiro, C.; Pedrol, N. On the bioherbicide potential of Ulex europaeus and Cytisus scoparius: Profiles of volatile organic compounds and their phytotoxic effects. PLoS ONE 2018, 13, e0205997.
- Kayanifard, M.; Mohsenzadeh, S. Allelopathic Analysis of Four Ecotypes of Ajowan. Iran. J. Sci. Technol. Trans. A Sci. 2017, 41, 971–978.
- Singh, H.P.; Batish, D.R.; Setia, N.; Kohli, R.K. Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann. Appl. Biol. 2005, 146, 89–94.
- Bernhoft, A. A Brief Review on Bioactive Compounds in Plants. In Bioactive Compounds in Plants—Benefits and Risks for Man and Animals; Bernhoft, A., Ed.; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010; pp. 11–17.
- Yasri, A.; Naboulsi, I.; Aboulmouhajir, A.; Kouisni, L.; Bekkaoui, F. Plants extracts and secondary metabolites, their extraction methods and use in agriculture for controlling crop stresses and improving productivity: A review. Acad. J. Med. Plants 2018, 6, 223–240.
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970.
- Echeng, F.; Echeng, Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020.
- Asplund, R. Monoterpenes: Relationship between structure and inhibition of germination. Phytochemistry 1968, 7, 1995–1997.
- Reynolds, T. Comparative Effects of Alicyclic Compounds and Quinones on Inhibition of Lettuce Fruit Germination. Ann. Bot. 1987, 60, 215–223.
- Dudai, N.; Ben-Ami, M.; Chaimovich, R.; Chaimovitsh, D. Essential oils as allelopathic agents: Bioconversion of monoterpenes by germinating wheat seeds. Acta Hortic. 2004, 629, 505–508.
- Elakovich, S.D. Terpenoids as Models for New Agrochemicals. In Biologically Active Natural Products; Cutler, H.G., Ed.; ACS Symposium Series; American Chemical Society: Hattiesburg, MS, USA, 1988; Volume 380, pp. 250–261.
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352.
- Stewart, A.J.; Stewart, R.F. Phenols. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2682–2689.
- Araniti, F.; Lupini, A.; Mercati, F.; Statti, G.A.; Abenavoli, M.R. Calamintha nepeta L. (Savi) as source of phytotoxic compounds: Bio-guided fractionation in identifying biological active molecules. Acta Physiol. Plant 2013, 35, 1979–1988.
- Eslahi, H.; Fahimi, N.; Sardarian, A. Chemical composition of essential oils: Chemistry, safety and applications. In Essential Oils in Food Processing: Chemistry, Safety and Applications; Hashemi, S.M.B., Khaneghah, A.M., Sant’Ana, A.S., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 119–171.
- Kordali, S.; Tazegul, A.; Cakir, A. Phytotoxic effects of Nepeta meyeri Benth. Extracts and essential oil on seed germinations and seedling growths of four weed species. Rec. Nat. Prod. 2015, 9, 404–418.
- Islam, A.M.; Hasan, M.; Musha, M.H.; Uddin, K.; Juraimi, A.S.; Anwar, P. Exploring 55 tropical medicinal plant species available in Bangladesh for their possible allelopathic potentiality. Ann. Agric. Sci. 2018, 63, 99–107.
- Scavo, A.; Restuccia, A.; Pandino, G.; Onofri, A.; Mauromicale, G. Allelopathic effects of Cynara cardunculus L. leaf aqueous extracts on seed germination of some Mediterranean weed species. Ital. J. Agron. 2018, 11, 119–125.
- Tigre, R.; Silva, N.; Santos, M.; Honda, N.; Falcão, E.; Pereira, E. Allelopathic and bioherbicidal potential of Cladonia verticillaris on the germination and growth of Lactuca sativa. Ecotoxicol. Environ. Saf. 2012, 84, 125–132.
- Issa, M.; Chandel, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Yadav, S.S.; Kumari, A. Appraisal of phytotoxic, cytotoxic and genotoxic potential of essential oil of a medicinal plant Vitex negundo. Ind. Crops Prod. 2020, 145, 112083.
- Dutra, Q.P.; Christ, J.A.; Carrijo, T.T.; Alves, T.D.A.; Alves, T.D.A.; Mendes, L.A.; Praça-Fontes, M.M. Phytocytotoxicity of volatile constituents of essential oils from Sparattanthelium Mart. species (Hernandiaceae). Sci. Rep. 2020, 10, 1–11.
- Duke, S.O.; Oliva, A. Mode of Action of Phytotoxic Terpenoids. In Allelopathy: Chemistry and Mode of Action of Allelochemicals; Macias, F.A., Galindo, J.C.G., Molinillo, J.M.G., Cutler, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 201–216. ISBN 0-8493-1964-1.
- McNeil, P.L.; Steinhardt, R.A. Loss, Restoration, and Maintenance of Plasma Membrane Integrity. J. Cell Biol. 1997, 137, 1–4.
- Pieracci, J.P.; Armando, J.W.; Westoby, M.; Thommes, J. Industry review of cell separation and product harvesting methods. In Biopharmaceutical Processing; Jagschies, G., Lindskog, E., Łącki, K., Galliher, P., Eds.; Elsevier: Cambridge, CA, USA, 2018; pp. 165–206.
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-Pinene Inhibits Growth and Induces Oxidative Stress in Roots. Ann. Bot. 2006, 98, 1261–1269.
- Varona, S.; Martín, Á.; Cocero, M.J. Formulation of a natural biocide based on lavandin essential oil by emulsification using modified starches. Chem. Eng. Process. Process. Intensif. 2009, 48, 1121–1128.
- Einhellig, F.A.; Rice, E.L.; Risser, P.G.; Wender, S.H. Effects of Scopoletin on Growth, CO2 Exchange Rates, and Concentration of Scopoletin, Scopolin, and Chlorogenic Acids in Tobacco, Sunflower, and Pigweed. Bull. Torrey Bot. Club 1970, 97, 22–23.
- Einhellig, F.A.; Rasmussen, J.A. Effects of three phenolic acids on chlorophyll content and growth of soybean and grain sorghum seedlings. J. Chem. Ecol. 1979, 5, 815–824.
- Pouresmaeil, M.; Nojadeh, M.S.; Movafeghi, A.; Maggi, F. Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed Convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Ind. Crops Prod. 2020, 155, 112785.
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic effect of two volatile monoterpenes against bill goat weed (Ageratum conyzoides L.). Crop. Prot. 2002, 21, 347–350.
- Vaid, S.; Batish, D.; Singh, D.H.; Kohli, R. Phytotoxic effect of eugenol towards two weedy species. Bioscan 2010, 5, 339–341.
- Chambers, E.E.; Holm, L.G. Phosphorus Uptake as Influenced by Associated Plants. Weeds 1965, 13, 312–314.
- Iii, C.E.O.; Rice, E.L. Relative Effects of Known Plant Inhibitors on Species from First Two Stages of Old-Field Succession. Southwest. Nat. 1970, 15, 165.
- Glass, A.D.M. Influence of Phenolic Acids on Ion Uptake: I. Inhibition of phosphate uptake. Plant Physiol. 1973, 51, 1037–1041.
- Glass, A.D.M. Influence of Phenolic Acids upon Ion Uptake: III. Inhibition of potassium absorption. J. Exp. Bot. 1974, 25, 1104–1113.
- Harper, J.R.; Balke, N.E. Characterization of the Inhibition of K+ Absorption in Oat Roots by Salicylic Acid. Plant Physiol. 1981, 68, 1349–1353.
- Glass, A.D.M. The allelopathic potential of phenolic acids associated with the rhizosphere of Pteridium aquilinum. Can. J. Bot. 1976, 54, 2440–2444.
- Greenland, D.J. Nitrate fluctuations in tropical soils. J. Agric. Sci. 1958, 50, 82–92.
- Cavalieri, A.; Caporali, F. Effects of essential oils of cinnamon, lavender and peppermint on germination of Mediterranean weeds. Allelopath. J. 2010, 25, 1–5.
- Al-Mariri, A.; Safi, M. In Vitro Antibacterial Activity of Several Plant Extracts and Oils against Some Gram-Negative Bacteria. Iran. J. Med Sci. 2014, 39, 36–43.
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement. Altern. Med. 2017, 17, 1–10.
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi, J. Biol. Sci. 2018, 25, 361–366.
- Menzies, J.D.; Gilbert, R.G. Responses of the Soil Microflora to Volatile Components in Plant Residues. Soil Sci. Soc. Am. J. 1967, 31, 495–496.
- Blum, U. Fate of phenolic allelochemicals in soils—The role of soil and rhizosphere microorganisms. In Allelopathy: Chemistry and Mode of Action of Allelochemicals; Macías, F.A., Galindo, J.C.G., Molinillo, J.M.G., Cutler, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 57–76.
- Emarmulla, R.; Eharder, J. Microbial monoterpene transformations—A review. Front. Microbiol. 2014, 5, 346.
- Alipour, M.; Saharkhiz, M.J.; Niakousari, M.; Damyeh, M.S. Phytotoxicity of encapsulated essential oil of rosemary on germination and morphophysiological features of amaranth and radish seedlings. Sci. Hortic. 2019, 243, 131–139.




