
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Outi Huttala | + 870 word(s) | 870 | 2020-04-16 11:27:05 | | | |
| 2 | Bruce Ren | Meta information modification | 870 | 2020-07-23 11:28:56 | | | | |
| 3 | Camila Xu | -1 word(s) | 869 | 2020-11-01 10:09:43 | | |
Video Upload Options
Vasculature interacts with variety of cell types in vivo and hence it can function as relevant component with various co-cultures. We have previously developed in vitro angiogenesis model. At the end point the cultures contain continuous vascular network spreading evenly across the well.
In vitro vascular structures formed from human adipose stromal cell (hASC) and human umbilical vein endothelial cell (HUVEC) co-culture were now scaled for various well plate formats. These included 48, 96 and 384. The vascular structures have been optimized and characterized previously on 48 well format. After scaling it was utilized for culturing cancer cell line cells and primary cancer cells from patients. They were also utilized to estimate the angiogenic induction potential of the primary patient derived cancer cells.
This scaling of the vascular structures allows better utilization of the in vitro vasculature. Now it can be utilized in high throughput applications. The applications of these in vitro vascular structures can include cancer research, cell biology, drug development and screening, and for personalized medicine.
1. Introduction
Vasculature interacts with variety of cell types in vivo and hence it can function as relevant component with various co-cultures. We have previously developed an in vitro angiogenesis model. At the end point the cultures contain continuous vascular network spreading evenly across the well plate well. The Vasculature has been previously utilized as a growth platform for cardiomyocytes [1] and adipocytes[2][3]. Blood supply is also essential for tumor growth and progression. It is needed to ensure the supply of nutrients and oxygen, but also to remove waste products from the tumor. Vasculature and its basement membrane are also important components to the tumor environment, taking part in for example in metastasis of cancer cells. In tumor-induced angiogenesis, tumor cells secrete angiogenic factors into surrounding normal tissue which, in turn, promotes the blood vessel formation into the tumor tissue. Due to the need of blood vessels, one effective mean to prevent growth and expansion of tumors is inhibition of angiogenesis. Angiogenesis inhibiting drugs are targeting various key phases of angiogenesis process including angiogenesis inducing growth factors such as VEGF, VEGF pathway, matrix metalloproteases and αvβ3-integrin–vitronectin interaction[4]. Due to the criticalness of crosstalk between cancer cells and their microenvironment, especially with cells forming the vasculature, we hypothesize that the traditional 2D culture can be greatly improved by adding vascular component to the culture.
In order to implement the developed cell assays in high throughput studies, the cell numbers were optimized for 48-, 96-, and 384-well plate formats. The cell densities of hASC and HUVEC (20000 and 4100 cells/cm2, respectively) were based on the previously published angiogenesis model, which has been intra-laboratory validated according to OECD guidance[5]. The vascular structures have been previously optimized and characterized on 48 well format[6].
For this entry [7], the vasculature was utilized as platformfor culturing cancer cells. Cell number optimization of cancer cells showed that most optimal cell numbers for cancer cells were 10000 cells per 48-well plate, 5000 cells per 96-well plate, and 1000 cells per 384-well plate. These cell numbers allowed enough room for cells to proliferate during the culture period and there were enough cancer cells compared to the number of vasculature cells, i.e., hASC and HUVEC. The 384-well plate used had square-shaped wells compared to the round-bottom well in 48- and 96-well plates. The cells in square wells tend to prefer growing in the middle of the well, and hence the optimal cell number/cm2 is smaller than in the 96-well plate. On the 48-well plate, 10,000 cancer cells occupied the whole well area evenly. This cell number was the highest possible, the cells detached from the well before day 7 when more than 10,000 cancer cells were plated. This was most likely due to an overgrowth of cells. It must be borne in mind that the vascular platform is alive with metabolically active dividing cells.
2. Discussion
The scaling of vascular structures to 48-well, 96-well, and 384-well plate sizes was successful in terms of formed continuous endothelial tubule network, visualized by von Willebrand factor (VWF) and intact basement membrane visualized by collagen IV (Figure 1). Hence the system is scalable to high throughput formats.
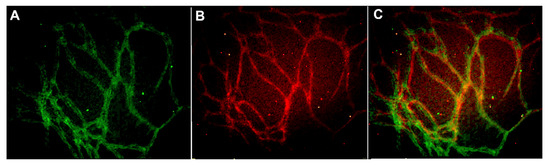
Figure 1. Vascular structures cultured on 384-well plate and stained with (A) anti-collagen IV (FITC-green) and (B) anti-von Willebrand factor (VWF) (TRITC-red) antibodies. The merged image is seen in (C). The edges and square shape of the 384 well plate caused some challenges in obtaining representative images from the formed vascular network. Image obtained with Cell-IQ with 10x objective.
After scaling it was utilized for culturing cancer cell line cells and primary cancer cells from patients. Due to the presence of vasculature in the co-culture of cancer cells and vasculature, the growth environment of cancer cells is closer to in vivo tumor microenvironment than in cancer cell monocultures. They were also utilized to estimate the angiogenic induction potential of the primary patient derived cancer cells.
Now that the protocol for producing in vitro vasculatur structures has been optimized i.e. scaled for various well plate formats it can be utilizes for various applications. The in vitro vasculature can be utilized as growth platform for example for cancer cells, adipocytes or cardiomyocytes. Applications of the co-cultures may include cell research, drug development and screening and personalized medicine
References
- Hanna Vuorenpaa; Liisa Ikonen; Kirsi Kujala; Outi Huttala; Jertta-Riina Sarkanen; Timo Ylikomi; Katriina Aalto-Setala; Tuula Heinonen; Novel in vitro cardiovascular constructs composed of vascular-like networks and cardiomyocytes. In Vitro Cellular & Developmental Biology - Animal 2013, 50, 275-286, 10.1007/s11626-013-9703-4.
- Outi Huttala; Maaria Palmroth; Pauliina Hemminki; Tarja Toimela; Tuula Heinonen; Timo Ylikomi; Jertta-Riina Sarkanen; Development of Versatile HumanIn VitroVascularized Adipose Tissue Model with Serum-Free Angiogenesis and Natural Adipogenesis Induction. Basic & Clinical Pharmacology & Toxicology 2018, 123, 62-71, 10.1111/bcpt.12987.
- Outi Huttala; Jertta-Riina Sarkanen; Tuula Heinonen; Timo Ylikomi; Presence of vasculature results in faster insulin response in adipocytes in vascularized adipose tissue model.. ALTEX 2019, 36, 419-434, 10.14573/altex.1811271.
- M K Oehler; Roy Bicknell; The promise of anti-angiogenic cancer therapy. British Journal of Cancer 2000, 82, 749-752, 10.1054/bjoc.1999.0991.
- T. Toimela; O. Huttala; E. Sabell; M. Mannerström; J.R. Sarkanen; T. Ylikomi; T. Heinonen; Intra-laboratory validated human cell-based in vitro vasculogenesis/angiogenesis test with serum-free medium. Reproductive Toxicology 2017, 70, 116-125, 10.1016/j.reprotox.2016.11.015.
- Outi Huttala; Hanna Vuorenpaa; Tarja Toimela; Jukka Uotila; Hannu Kuokkanen; Timo Ylikomi; Jertta-Riina Sarkanen; Tuula Heinonen; Human vascular model with defined stimulation medium – a characterization study. ALTEX 2015, 32, 125-136, 10.14573/altex.1411271.
- Outi Huttala; Synnöve Staff; Tuula Heinonen; Johanna Mäenpää; Minna Tanner; Timo Ylikomi; In Vitro Vascular Network Modified to Function as Culture Platform and Angiogenic Induction Potential Test for Cancer Cells. International Journal of Molecular Sciences 2020, 21, 1833, 10.3390/ijms21051833.




