
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Irina Kurzina | + 1375 word(s) | 1375 | 2021-03-16 09:28:38 | | | |
| 2 | Rita Xu | Meta information modification | 1375 | 2021-03-17 09:21:57 | | |
Video Upload Options
The promising techniques of obtaining AO adsorbents are discussed, namely the technique of thermal activation in the mode of pneumatic transport with gibbsite by heated air (TCA Gb) and the technique of thermal activation of gibbsite in centrifugal flash reactors (CTA Gb).
1. Introduction
Adsorbents-desiccants of the air are successfully applied in different fields of industry, particularly in mechanical engineering and for the elimination of excessive moisture in amenity premises. As a rule, they are also used at a dewpoint for compressed air below 0 °C. The adsorption method is applied in processes in which a high degree of air dehydration is required (pharmaceutics, chemical and petrochemical plants, textile factories, food and electronic industry, etc.) [1][2][3].
The following requirements are imposed on industrial adsorbents-desiccants [4][5]:
- -
-
Interaction processes between adsorbents and water vapours must be fast. Adsorbents must have high absorption capacity, which will allow the gas to pass through adsorbers at a high rate and use compact adsorption plants for dehydration.
- -
-
Adsorbents must have gigh stability after multiple regenerations.
- -
-
Adsorbent grains must have high mechanical compression, (crushing) and abrasion strength.
- -
-
Adsorbents must be inexpensive and easily regenerated.
- -
-
Adsorbents must not react chemically during adsorption and regeneration.
To meet the abovementioned requirements for the efficiency of the adsorbent, it must have the following characteristics:
- -
-
Large internal pore volume
- -
-
Large value of specific surface
- -
-
Controlled pore-size distribution, preferably in the micropore range
- -
-
Controlled properties of the surface, owing to selected functional groups
- -
-
Weak interactions between an adsorbate and an adsorbent (in general, physical adsorption)
Molecular sieves (zeolites), silica gels, and activated aluminium oxide are usually used as adsorbents-desiccants. These adsorbents have their own advantages and disadvantages [5][6][7][8][9][10][11][12][13], as shown in Figure 1.
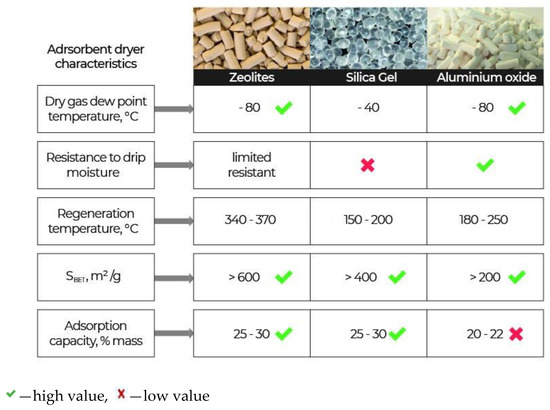
Figure 1. Characteristics of the basic adsorbents-desiccants.
Zeolites have sufficiently large adsorption capacity by water, but they are costly and difficult to regenerate. Prolonged time and temperatures above 340 °C are required for their regeneration. A distinctive feature of zeolites is a high adsorption rate of water vapour (steep rise of isotherms) in the area of low concentrations of water vapour. The amount of the absorbed water reaches a maximum value with a relative humidity equal to 20% and practically remains constant with a subsequent increase in the air humidity [14]. The second distinctive feature of adsorption of water vapour on zeolites is a weak dependence of its adsorption capacity on the temperature. At a temperature of 100 °C and a pressure of 10 mm of mercury, the adsorption capacity of zeolites reaches 15 ÷ 16 g/100 g of the adsorbent. Even at 200 °C, it is still significant at—3.6 g/100 g of the adsorbent [14].
2. Methods of Obtainment of Aluminium Oxide Used in Industry
As a rule, bauxite, alunite and nepheline are used as raw materials for obtaining aluminium oxide. If the content of aluminium oxide in them is more than 6−7%, the production is carried out by the main method—the Baeyer method. If the content of the substance is lower, the method of sintering ore with lime or soda is used. The main raw-material source of Al2O3 is bauxite, which mainly consists of hydrated forms of Al2O3 (gibbsite, boehmite, diaspore). On average, bauxite contains from 45 wt.% to 60 wt.% of Al2O3 (in conversion from hydroxides), 10–30 wt.% of Fe2O3 and varying amounts of SiO2, CaO, TiO2 and H2O.
2.1. Methods of Alumina Obtainment
2.1.1. Bauxite Ore Treatment by the Baeyer Method
As of today, about 90–95% of the world’s aluminium hydroxide is extracted using the Baeyer process, which was proposed in 1887 [15]. The Baeyer method is a hydrochemical method of obtaining alumina from bauxites. Large pieces of bauxite, supplied from the mines, are first crushed, and then wet-ground in ball mills. Bauxite, caustic alkali and recycled liquor are fed to ball mills. Sometimes, a little lime is added to bauxite to facilitate their breakability. The pulp from the mills is collected in collectors, where the remaining amount of the recycled liquor with a concentration of Na2O = 300 g/l is fed. Then, it is heated there by separation steam to 90–100 °C and soaked for 4–8 h while stirring for preliminary bauxite desiliconization, i.e., there is a transfer of the most part of the active silica from bauxite to the liquor. The mixture of the ground bauxite and the recycled liquor (raw pulp) is sent to one of the main operations. This process is called the leaching or cooking of bauxite, which is carried out in autoclaves at a temperature of 230–240 °C. The purpose of this operation is to dissolve the aluminium oxide contained in bauxite, avoiding the transfer of other components of bauxite (silica, iron oxides, etc.) to the liquor. The silica is removed by subsequent slow heating when Na2Si(OH)6 precipitates. The remaining pure liquor of NaAl(OH)4 is cooled, diluted with water and neutralized with carbon dioxide. As a result, aluminium trihydrate Al(OH)3 (gibbsite) is selectively deposited from the liquor without the residues of the dissolved silica.
2.1.2. Sintering Technique
The technique of sintering ore with lime or soda involves mixing high-silica finely ground ore (nepheline and others) with soda and limestone and sintering in rotating furnaces at 1250−1300 °C. The obtained mass is leached with an aqueous alkaline solution. The solution of sodium aluminate, obtained in this way, is separated from the sludge, then it is released from SiO2, precipitating it in an autoclave at a pressure of about 0.6 MPa and then with lime under atmospheric pressure, and the aluminate is decomposed with gaseous CO2 along with the formation of Al(OH)3.
Both described methods are multistage. They include both the main technological stages of production and auxiliary operations related to waste disposal and to the repeated return of mother liquors to the circulating cycle [16]. The main product when using the abovementioned methods of ore processing is gibbsite (hydrargillite).
2.2. Methods of AO Obtainment
To obtain active aluminium oxide, various methods of processing gibbsite are used [17][18], as described in the following subsection.
2.2.1. Aluminate Technology with Alkali Treatment
- (a)
-
Dissolution of gibbsite in alkali, accompanied by the formation of sodium aluminate:
-
(b)
-
Reprecipitation with acid:
A continuous process based on the reaction of HNO3 with NaAlO2 was described by the authors of [18]. The principle is as follows: In the first reactor, HNO3 and NaAlO2 are mixed at a temperature of 30 °C to 75 °C. Then, the resulting suspension is sent to the second reactor where it is converted into pseudoboehmite. The suspension fraction is recycled in the first reactor with a ratio of 0.1 to 3 of slurry volume per volume of mixing (NaAlO2 + HNO3). Pseudoboehmite is then removed from the second reactor. After drying, it has a specific surface area in the range of 200 m2/g to 300 m2/g.
This method is the most common for producing aluminium gel for catalysis. Precipitation is carried out from alkaline solutions (aluminates) with acids (sulfuric, nitric, hydrochloric) or acidic solutions of salts. Various structural and texture characteristics of the resulting hydroxide are determined by the pH, temperature and nature of the anion. The crystallisation rate of pseudoboehmite is determined largely by the temperature of deposition, and bayerite is primarily determined by the pH.
To increase the dissolution velocity, gibbsite is preliminary ground to the particle size of 10 µm and/or the temperature of the reacting mixture is raised.
There are various techniques of precipitation—with a variable and constant value of pH, two-stage (cold and hot precipitation), etc. For example, cold precipitation from the sodium aluminate solution with the sulphuric acid solution is carried out at 20–25 °C and pH = 9.3–9.5. Hot precipitation is performed at 90–95 °C and pH = 9.3–9.5. Then, both modifications of aluminium oxide are mixed, and a precipitate consisting of pseudoboehmite and boehmite is obtained, which can be rinsed and filtered very well. Granules of active aluminium oxide of high mechanical strength can be obtained using this technique.
The drawback of this method is the high cost of removing sodium due to the difficulty of rinsing the gel. The aluminium hydroxide precipitate is filtered, rinsed on a filter press and moulded into granules, which are later dried and calcined at 670–820 K to obtain η- or γ-Al2O3.
References
- White, D.H. Compressed Air and Gas Purification and Fractionation for High Purity Applications by Improved PSA Processes. Sep. Sci. Technol. 2008, 43, 2298–2306.
- Achenbach, K. Dehumidification. Planning Guidelines for Technical Building Services and Specialist Planners. 2016. Available online: https://www.condairgroup.com/m/0/planningbrochure-dehumidification-161202-en.pdf (accessed on 23 December 2020).
- Atlas Copco. White Paper—Compressed Air Drying. 2016. Available online: https://www.atlascopco.com/content/dam/atlas-copco/compressor-technique/oil-free-air/documents/Compressed_air_drying_whitepaper_EN_Antwerp_2937016013.pdf (accessed on 23 December 2020).
- Stewart, M. Dehydration. In Surface Production Operations: Design of Gas-Handling Systems and Facilities; Gulf Professional Publishing: Houston, TX, USA, 2014; Volume 2, pp. 279–373. ISBN 9780123822086.
- Shuguang, D. Sorbent Technology. Encyclopedia of Chemical Processing. 2006. Available online: https://www.researchgate.net/publication/267778978_Sorbent_Technology (accessed on 23 December 2020).
- Ciahotný, K.; Hlinčík, T.; Vagenknechtová, A.; Prokeš, O. Adsorbents for the natural gas drying at CNG stations. Acta Montan. Slovaca 2016, 21, 306–313. Available online: https://actamont.tuke.sk/pdf/2016/n4/6ciahotny.pdf (accessed on 23 December 2020).
- Linsen, B. Physical and Chemical Aspects of Adsorbents and Catalysts; Academic Press: London, UK; New York, NY, USA, 1970; ISBN 0124511503.
- Desai, R.; Hussain, M.; Ruthven, D.M. Adsorption of water vapour on activated alumina: I—Equilibrium behaviour. Can. J. Chem. Eng. 1992, 70, 699–706.
- Rudisill, E.N.; Hacskaylo, J.J.; LeVan, M.D. Coadsorption of hydrocarbons and water on BPL activated carbon. Ind. Eng. Chem. Res. 1992, 31, 1122–1130.
- Rege, S.U.; Yang, R.T.; Buzanowski, M.A. Sorbents for air prepurification in air separation. Chem. Eng. Sci. 2000, 55, 4827–4838.
- Kim, J.-H.; Lee, C.-H.; Kim, W.-S.; Lee, J.-S.; Kim, J.-T.; Suh, J.-K.; Lee, J.-M. Adsorption of water vapour on alumina, zeolite 13X and a zeolite X/activated carbon composite. J. Chem. Eng. Data 2003, 48, 137–141.
- Serbezov, A. Adsorption equilibrium of water vapour on F-200 activated alumina. J. Chem. Eng. Data 2003, 48, 421–425.
- CECA. Molecular Sieves. General Technical Information. 2015. Available online: https://www.cecachemicals.com/export/sites/ceca/.content/medias/downloads/products/dtm/brochure-gti-siliporite-2015_bdef.pdf (accessed on 23 December 2020).
- Keltsev, N.V. Fundamentals of Adsorption Technology; Chemistry: Moscow, Russia, 1984; p. 595.
- Abuzov, A.M. Aluminium Oxide and Alumina Ceramics (review). Part 1. Properties of Al2O3 and Commercial Production of Dispersed Al2O3. Refract. Ind. Ceram. 2019, 60, 24–32.
- Wasserman, I.M. Chemical Deposition from Solutions; Chemistry: Leningrad, Russia, 1980; p. 208.
- Stiles, A.B. Catalyst Supports and Supported Catalysts: Theoretical and Applied Concepts; Butterworth-Heinemann: Boston, MA, USA, 1987; p. 270. ISBN 9780409951486.
- Schüth, F.; Sing, K.S.W.; Weitkamp, J. Handbook of Porous Solids; Wiley-Vch: Weinheim, Germany, 2002; pp. 1591–1677. ISBN 9783527302468.




