1000/1000
Hot
Most Recent

Chitosan (CS) has been widely used as a surface coating for metal nanoparticles. CS can work as a reducing agent, a shape director, or a size-controllable agent in the synthesis of metal nanoparticles. The previous studies have shown that functionalizing the surface of metal nanoparticles by CS can offer many advantages, including improving physicochemical stability, a drug carrier, controlling drug release, promoting muco-adhesiveness and tissue penetration, encouraging cell interactions, and enhancing antimicrobial effects.
Chitosan (CS) is considered as a non-toxic, odorless, biocompatible, and biodegradable biopolymer. Thus, CS is considered a renewable, sustainable, and affordable polymer . In the structure of CS (Figure 1), an amino group together with hydroxyl groups (both principal and secondary ones) are the reactive functional groups . The structure of CS and its physicochemical properties vary with the amino group through intra-molecular and inter-molecular hydrogen bonds. The molecular weight (MW, the number of sugar units over polymeric molecule) and the degree of deacetylation (DDA) are known as the main parameters that affect the CS’s properties . As reported in References [1], the physicochemical characteristics, including solubility, adsorption on solids, tear strength, viscosity, elasticity, and bio-functional activities are strongly associated with the polymeric MW. The DDA affects the solubility of CS in the acid solution and the flexibility of CS molecules [2]. The CS with high DDA is more flexible and tends to shape an irregular coil with additional intramolecular hydrogen bonds inside the CS chain . Consequently, the CS chain becomes less intertangled in the structure and has a more elliptical shape. The mechanical properties of the elliptical CS chain are generally less strong than those of less deacetylated microspheres in general. On the other hand, the CS chain with less deacetylation is more expanded and has stronger interactions between molecules, making the chain more intertangled. The DDA also plays an important role in the proliferation and adhesion of cells, as reported in References [3][4][5][6]. The CS with low DDA is favorable for the growth and adhesion of cells. The viscosity of the CS mixture decreases with the temperature and increases with the DDA and the concentration. The solubility of CS is dependent on the pKa and the acidic solvent’s strength. CS is soluble in a weak acidic solution, but insoluble for pH > 7.
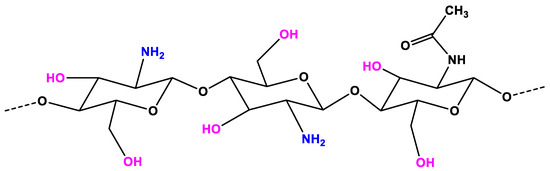
Figure 1. The chemical structure of chitosan (CS).
CS is the second most abundant natural polymer and is the only natural polycation alkaline polysaccharide with a glucosamine content of more than 90% [7]. They are easy to be extracted from natural sources [8][9] and have a cheap price in comparison with some other polymers, such as fucoidan or hyaluronan acid. The glucosamine backbone of CS contains a high density of the amino [10], which makes CS become a very bioactive polymer. CS can be modified to enhancing the desired properties [11]. With those excellent properties, CS is a polymer that has been applied in the green preparation of metal nanoparticles for biomedicine application.
The antimicrobial activity of CS has been proven by many studies [12]. The inhibition mechanism is based on the interaction of the positive charge CS at the acidic condition with negatively charged residues of biomolecules on the surface of the bacteria cell . Another possibility is that CS permeates into the cell nucleus and inhibits the RNA and protein synthesis as well as the rupture and leakage of the intracellular component [13]. Pedro et al. [14] demonstrated that electrostatic interaction of CS with polar groups dipalmitoyl phosphatidylglycerol of bacterial membranes are dominant, resulting in changes of membrane potential, elasticity, and possibly its permeability to biomolecules. In contrast, the interaction of CS and the dipalmitoyl phosphatidylcholine monolayer of mammalian cell membranes are weak and likely favored by hydrophobic interactions of the CS backbone with lipid tails. These different interaction mechanisms may explain why CS-based material are usually bactericide, but not toxic to mammalian cells.
The MW strongly affects the antibacterial properties of CS [15]. CS with low MW penetrates easily to the cell wall of bacteria. Meanwhile, the CS with high MW has a lower permeation capacity to the bacterial membrane. The antimicrobial activity of CS is also influenced by other factors such as solubility, pH, and the temperature environment.
The free radical reaction contributes to many chronic health problems [16]. Free radicals are unstable and tend to pair up with other molecules and atoms to form a stable state. Antioxidants can prevent the formation of free radicals by scavenging them, or by promoting their decomposition. Recently, the antioxidant activity of CS has been investigated by many research groups [17][18][19]. CS exhibits outstanding scavenging activity against different radical species. The mechanism of the antioxidant property of CS is based on the donating hydrogen atoms for free radicals binding [20]. Mahdy Samar et al. [21] tested an antioxidant activity of CS with various DDA and MW and observed that CS with high DDA and low MW has better antioxidant activity.
From the experimental results, many studies have confirmed that CS has anti-inflammatory and anti-proinflammatory properties. Davydova et al. [22] tested the anti-inflammatory activity of CS with high (115 kDa) and low MW (5.2 kDa). Both CS samples induced the anti-inflammatory in animal blood and suppression of colitis progress. The results showed that the anti-inflammatory activity of CS depends on structural elements. Meanwhile, the MW does not affect this activity. However, Chang et al. [23] claimed that larger MW (>29.2 kDa) CS has anti-inflammatory activity whereas smaller MW (≤29.2 kDa) CS have proinflammatory activity. Despite those previous research attempts, extensive studies still need to be carried on to understand the anti-inflammation of CS.
Many reports showed that CS can be a potential anti-cancer polysaccharide [24][25][26][27] It can inhibit tumor growth by preventing tumorigenesis, inducing tumor cell apoptosis, and inhibiting tumor metastasis. Through the experimental evaluation, Park et al. [28] concluded that the MW and DDA of CS are important factors for the exhibition of anti-tumor activity in vitro. The experiments on three cancer cell lines pointed out that the CS with lower MW and higher DDA has better anti-cancer activity. Another study [29] showed that CS has a larger effect on a negatively charged tumor cell. Particularly, the decreased level of vascular endothelial growth factor receptor 2 on the tumor surface limited the growth of HepG2 cells and led to the inhibition of tumor angiogenesis.
CS can affect both the formation and the functionalization processes of metal nanoparticles (Figure 2). When the cationic polymer CS is added to the reaction solution, electrostatic interaction happens between positively-charged CS with negatively-charged nanoparticles (which have the negative capping agent) [30], or the interaction occurs by absorbing CS on the metal nanoparticle surface [31], resulting in a CS shell around the nanoparticles. Regarding the formation process, the CS can add before or during the formation of the nanoparticles. Thus, CS directly affects the formation of nanoparticles. In this case, CS can act as the stabilizing agent, the reducing agent, the size-controllable agent, and the shape-direction agent for the synthesis of metal nanoparticles. For the functionalization process, CS is used to modify the surface of nanoparticles to enhance the biocompatible and carrying abilities for drugs of metal nanoparticles.
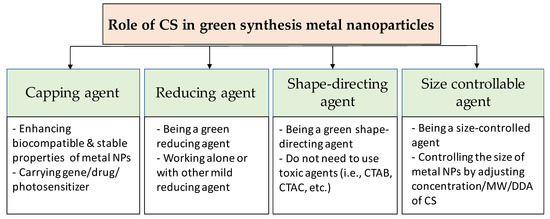
Figure 2. Overview of the role of chitosan in green synthesis of metal nanoparticles.
The natural polymers are usually chosen as a stabilizer for the synthesis of metal nanoparticles. With the availability, biocompatibility, highly positive charge, CS becomes a good stabilizer of metal nanoparticles. As shown in Figure 3a, the bare metal nanoparticles can easily aggregate in solution due to the Van der Waals interactions between raw metal surfaces. In contrast, CS is a steric barrier with a positive charge density covered around the metal. The strong electrostatic interaction among positive-charged metal nanoparticles allowed the formation of homogeneous metal nanoparticle solutions, as seen in Figure 3b. Many studies reported the good performance of CS in the role of stabilizing metal nanoparticles. For example, CS was utilized as the stabilizer to synthesis the silver nanoparticles (AgNPs) using a green approach based on an electrochemical oxidation/complexation process with UV irradiation reduction [32]. In another example, gold nanoparticles (AuNPs) were synthesized in the presence of CS and citric acid. The as-prepared AuNPs were stable in the aqueous phase without any agglomeration [33]. The water-soluble chitosan oligosaccharide (COS) from CS has also been widely used for coating metal nanoparticles oriented toward particular biomedical applications, including drug/gene delivery, photo-based therapy, and tissue engineering . The COS was used as a green reducing agent/stabilizer for the one-pot synthesis of AuNPs for gene transfer [34]. The positive charges by the amino groups of COS improved the affinity with plasmid DNA.
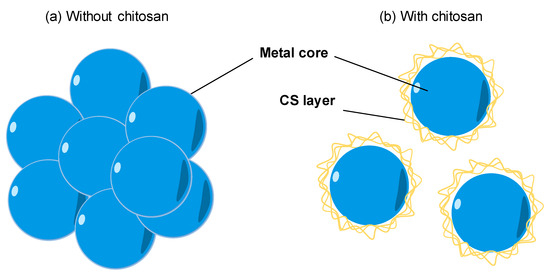
Figure 3. The effect of chitosan (CS) capping on the dispersion of metal nanoparticles. (a) The aggregation of metal nanoparticles without a CS capping agent in solution. (b) The good dispersion of metal nanoparticles with a CS capping agent in solution.
The toxic reducing agents are often used in the synthesis of nanoparticles by chemical methods, which release environmentally hazardous chemicals. The nanoparticles synthesized from chemical routes could not be directly used for biomedical applications because of the presence of toxic capping, which cannot be separated easily from the nanoparticles. To protect the environment and to be used in biomedicine, nanoparticles need to be synthesized through green methods and with green materials. Many reports have revealed that CS can act as both reducing and stabilizing agents for the green synthesis of AgNPs [35][36], copper nanoparticles (CuNPs) [37][38], and AuNPs [36][39][40]. Carapeto et al. [41] put the effort to unravel the reaction mechanism of Ag ion by CS with the help of UV/Vis absorption and x-ray photoelectron spectra analysis. The experimental results showed that the very fast Ag reduction in CS aqueous solutions happens in the early stages even at room temperature, and the reaction happens faster when the temperature reaction was increased. The oxidation of alcohol or glucosidic groups of several functional groups in CS provides the free electron to reduce Ag+ to Ag0 and to form the carbonyl groups. By the UV-Vis spectra (absorption peak at ~262 nm, which indicates π* ← n transition in a carbonyl group) and the X-ray photoelectron spectroscopy (XPS), the authors proved that the main products in the reaction medium are carbonyl groups. The coating/wrapping of the metal with the cationic CS results in the positively charged nanoparticles and long-term stability in terms of aggregation [42].
Wongpreecha et al. [43] provided another explanation for the reaction mechanism of CS. The green process was taken in an autoclave under high temperature (120 °C) and high pressure (15 psi). In a pH 4 environment (lower than pKa of CS), Ag+ was coordinated with pair electrons of nitrogen and/or oxygen on the CS backbone. Then, Ag+ was reduced to Ag0 by a lone pair electron of oxygen in CS under high temperature and high pressure. CS played as a steric and electrostatic stabilizer for the resulting nanoparticles.
Creating a core-shell nanostructure is an effective strategy to enhance the performance of metal nanoparticles. The CS was also used as a green reducing agent to fabricate nanoparticles with the core-shell nanostructure for applications in the biomedicine field. Wang et al. [44] prepared a core-shell nanocomposite termed Cu@Pd-CS by the green method with natural CS. The synthesized Cu@Pd-CS showed good stability, sensitivity, and anti-interference.
Another role of CS in the green synthesis of metal nanoparticles is size control. Based on the UV–visible absorption spectrum data, Kalaivani et al. [45] observed that the AgNPs formation was efficiently increased in the presence of CS. In addition, the size of AgNPs was remarkably decreased at a higher CS concentration (Figure 4). This conclusion was again confirmed by our recent studies. The size of metal nanoparticles (i.e., PdNPs [46] and AuNS [47]) was decreasing when the added concentration of CS was increased. We proposed a hypothesis to explain how the CS affects the size of formed nanoparticles. During the formation of metal nanoparticles in the presence of CS, the positively charged CS has a strong electrostatic interaction with the metal nuclei. The higher concentration of CS leads to the stronger interaction of CS and the metal nuclei. This strong interaction inhibits the binding of precursors to the metal nuclei. Thus, metal nuclei are not able to grow more in the presence of a high concentration of CS solution.
Figure 4. The correlation of concentration of chitosan and the size of metal nanoparticles.
In the chemical route, toxic capping agents (e.g., , trisodium citrate) are usually used as the shape-directing agent for the metal nanoparticles. Replacing them with natural products is a good strategy to enhance the biocompatibility of metal nanoparticles for sustainable biomedical applications. CS was used as a structure-directing agent for the electrodeposition of AgNPs on disposable, pencil graphite electrodes [48]. The authors observed that AgNPS has well-defined morphologies in the presence of CS. Meanwhile, AgNPs have an irregular structure in the absence of CS. By controlling the experimental conditions, various morphologies of Ag NPs such as hexahedron, leaf, and dendrites have been obtained.
In addition, the properties of CS for controlling the shape of nanoparticles can be enhanced by modifying the CS, such as by using an anionic ligand, o-carboxymethyl, or a cationic N-trimethylamine group. Different shapes of AuNPs can be formed by the controlling effect of the CS’s positive and negative charges. As a soft template, thiolate-functionalized CS can be used for the preparation of gold nano chains, nanoneedles, as well as nanoflowers, as reported in Reference [49]. The CS’s thiolgroup can extensively interact with AuNPs owned by various assemblies. Additionally, it is shown that a peptide that contains an aromatic moiety can be used to template the architecture of gold nanocrystal via self-build, as reported in Reference [50]. In another study [51], folic acid (FA) and gallic acid (GA) -N-trimethyl CS (FA-GA-TMC) was demonstrated for the self-assembly of SeNPs with a cubic shape. With the modification of CS, three important structural features can be obtained, as follows: (1) the improvement of the electrostatic interaction between the negatively charged surface of SeNPs [52] and the stabilizer’s positive charge due to the N+(CH3)3 group’s positive charge of the quaternized CS, (2) the creation of a π-π stacking interaction together with a rigid template through hydrophobic elements of both the , and (3) the last one is the hydrogen bonding groups from the GA, FA, and the CS backbone. The interaction between the hydrophilic group of N+(CH3)3 and the negative charge of the SeNPs’ surface creates an outward presentation of the large hydrophobic groups of the GA and FA. This allows the π-π stacking interactions and the hydrogen-bonding interactions among surrounding particles, and further open the door for the assembly into cubic-like SeNPs.