1000/1000
Hot
Most Recent

The following paragraph resumes and accurately describes the process of metastasis, focusing on the breast to bone one. We will briefly illustrate the composition of the pre-metastatic niche and explain the involment of healthy bone cells in the metastasis establishment. This short intro is extracted from a review article which aim is to discuss the current available 3D models used to study the metastasis process.
The ability of cancer cells to leave a primary tumor, to disseminate through the body, and to seed new secondary tumors is universally recognized to be the basis for metastasis formation. Various and sometimes conflicting hypotheses have been proposed to explain different aspects of this process, but no single concept unravels the mechanism of metastasis in its completeness.
A pioneer study conducted by Stephen Paget in 1889 hypothesized that metastasis is based on the interplay between the so-called ‘seeds’ (namely the cancer cells) and the ‘soil’ (or the host microenvironment) [1]. Paget’s theory was subsequently challenged by others studies, such as Ewing’s and Isaiah Fidler’s research [2][3]. Other important findings revealed that primary tumors themselves are responsible for the formation of suitable microenvironmental conditions in distant sites, being determinant for the sustainment of cancer cells survival and proliferation before the establishment of the new colony [4][5][6]. Nowadays, this particular microenvironment is usually referred to as ‘pre-metastatic niche’ [5][7]. The metastatic niche theory suggests that a properly favorable microenvironment (pre-metastatic niche) supports tumor cells to engraft (metastatic niche) and proliferate at secondary sites (micro- to macrometastatic transition). In particular, the pre-metastatic niche results from the synergic interaction between the endogenous organ microenvironment and specific factors secreted by primary tumors [7][8].
The bone niche is populated by different kind of cells including stem cells, progenitor cells, mature immune cells, and supporting stromal cells [9][10][11]. To date, two primary niches have been described, namely the osteoblastic niche and the perivascular one; they are characterized by two diverse types of adult stem cells and their progeny: hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) [11][12][13].
HSCs are multipotent progenitor cells that can be found in adult bone marrow, peripheral blood, and umbilical cord blood. The hierarchical lineages of HSCs consist of myeloid cells, B lymphocytes, and osteoclasts [14]. The MSCs are multipotent cells that are able to differentiate into the mesenchymal lineage cells, which include osteoblasts, adipocytes, chondrocytes, fibroblasts, and other stromal cells [11][12]. Both cells’ lineages are connected to each other in the bone niche and work together to maintain bone homeostasis, sustaining in particular the osteogenesis, osteoclastogenesis, and hematopoiesis processes.
Bone is a hierarchically organized connective tissue; it contains four types of cells—osteogenic cells, osteoblasts, osteocytes, and osteoclasts—embedded in a matrix of collagen fibers and hydroxyapatite, as an inorganic component. Osteogenic cells differentiate into osteoblasts [15]. When included into the calcified matrix, osteoblasts undergo their terminal differentiation into osteocytes, changing their structure and function. On the other hand, osteoclasts are large multinucleated cells derived from the hematopoietic lineage (monocytes). Both osteoblasts and osteoclasts participate in the maintenance of bone physiologic homeostasis; in fact, bone tissue is continuously remodeled in order to maintain structure and calcium equilibrium, by osteoclast-mediated bone resorption and osteoblast-mediated bone deposition [16].
In case of cancer progression, this equilibrium is usually altered, leading to osteoblastic, osteolytic, or mixed metastatic lesions depending on the cancer origin and type [16]. In osteoblastic metastasis, commonly found in PC patients, the metastatic bone is characterized by the deposition of new tissue not preceded by bone resorption, resulting in excessive and disorganized bone formation [16]. Instead, osteolytic metastasis is mainly diagnosed in breast, lung, and renal cancers, and it is usually present uncontrolled osteoclast activity [15]. In most of the cases, the two processes coexist; thus, is not possible to classify bone metastasis as a single defined process, with the clinical prevalence of one over the other [17].
The development of malignant bone metastasis is recognized as a dynamic multistep process, in which a subpopulation of cancer cells from the primary tumor gain the capability to invade surrounding tissues, intravasate, survive in the bloodstream, and extravasate, giving rise to the metastatic colonization in a distant bone microenvironment [18]. Considering its complexity, a successful approach to study the intrinsic biology of bone metastasis is to separate the various steps of the cascade and to deeply characterize the bone metastatic microenvironment. Quiao and Tang extended the concept of “fertile soil“, proposed by Paget, to include three distinct microenvironments: the primary tumor microenvironment (PTM), the circulation microenvironment (CM), and the bone microenvironment (BM), each one distinguished by several key points that will be further discussed [19].
In the context of the PTM, the formation of metastasis involves a subgroup of osteotropic cancer cells with increased proliferative and migratory capacities [19]. Angiogenesis and epithelial to mesenchymal transition (EMT) are critically important in this phase. The first responds to the increased metabolic demand of cancer cells supporting local invasion and distant dissemination [20]. In contrast, the EMT consists of the cellular transformation from an epithelial phenotype with apical–basal polarization to a mesenchymal one characterized by high motility features [21]. The activity of cancer cells in the CM begins with intravasation and ends with extravasation. Tumor cells that trespass the normal vascular endothelium using the newly formed microcapillaries, become circulating tumor cells (CTCs), and invade the CM. Due to the overexpression of various surface receptors involved in pro-survival pathways [22], these CTCs are able to evade anoikis and survive in the CM. Once CTCs enter the BM, they are redefined as disseminated tumor cells (DTCs); here, DTCs can remain in a dormant state for several years [23]. This condition, also known as “dormancy”, can revert in case of stress circumstances, compromised immune system, and/or the activation of specific molecular pathways, leading to the formation of macrometastases. When in the osseous tissue, CTCs influence the pre-metastatic niche already developed in order to create a compatible niche to support the metastatic growth (metastatic niche). This process has been largely studied particularly for BC and PC bone metastasis and reproduced in the up-to-date available 3D model of bone metastasis formation [24] (Figure 1).
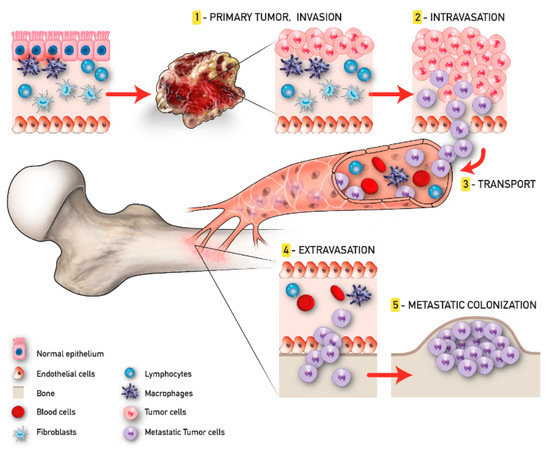
Figure 1. Tumor progression and bone metastasis process.