1000/1000
Hot
Most Recent

Cancer is characterized by unrestricted cell proliferation, inhibition of apoptosis, enhanced invasion and migration, and deregulation of signaling cascades. These properties lead to uncontrolled growth, enhanced survival, and the formation of tumors. Carnosol, a naturally occurring phyto-polyphenol (diterpene) found in rosemary, has been studied for its extensive antioxidant, anti-inflammatory, and anticancer effects. In cancer cells, carnosol has been demonstrated to inhibit cell proliferation and survival, reduce migration and invasion, and significantly enhance apoptosis. These anticancer effects of carnosol are mediated by the inhibition of several signaling molecules including extracellular signal-regulated kinase (ERK), p38, c-Jun N-terminal kinase (JNK), Akt, mechanistic target of rapamycin (mTOR) and cyclooxygenase-2 (COX-2). Additionally, carnosol prevents the nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and promotes apoptosis, as indicated by increased levels of cleaved caspase-3, -8, -9, increased levels of the pro-apoptotic marker Bcl-2-associated X (BAX), and reduced levels of the anti-apoptotic marker B-cell lymphoma 2 (Bcl-2).
Cancer is characterized by the unregulated proliferation of cells, the inhibition of programmed cell death—known as apoptosis—altered metabolism, tissue invasion and metastasis, and dysregulation of cell signaling that ultimately leads to enhanced survival, growth, and tumor formation [1]. Mutations and epigenetic changes allow cancer cells to grow and replicate uncontrollably and invade normal tissues [2]. This uncontrolled growth and proliferation arises due to loss of function mutations in tumor suppressor genes such as TP53 which encodes the tumor suppressor protein p53, as well as, mutations converting proto-oncogenes to oncogenes which lead to the hyperactivation of signaling pathways that promote growth and survival such as the PI3K-Akt pathway and the Ras-ERK pathway [2].
Despite the established treatments of cancer that include surgery, chemotherapy, and radiotherapy, cancer-related deaths are on the rise globally [3]. Novel plant-derived chemicals may provide an alternate effective method for the treatment of cancer. For much of history, people have used plants and plant extracts to treat their ailments [4]. Outside the Western world, phytotherapy still remains very common, and has since gained popularity in the West, with an estimated 30% of Americans currently using plant-based remedies [5]. Furthermore, many established pharmaceuticals are derived from plants, such as metformin, which was isolated from French lilac, morphine from the opium poppy, and aspirin from willow tree bark [6]. Developing an understanding of plant extracts and plant-derived compounds continues to provide great opportunities to assess and establish new chemicals for preventative and therapeutic purposes.
Polyphenols are a class of compounds found abundantly in many plant species that are regularly consumed by humans [7]. These compounds are partially responsible for the colour, fragrance, and taste of many fruits, vegetables, and herbs. Many polyphenols possess a bitter, astringent flavour often associated with foods such as nuts, tea, coffee, cider, and wine [8]. However, the properties of polyphenols go beyond just the taste and smell of food as they have been found to possess significant antioxidant, anti-inflammatory, cardioprotective, neuroprotective, antidiabetic, and anticancer properties [9][10][11][12]. Therefore, the study of natural products and plant-derived polyphenols is important as it provides opportunities to uncover the therapeutic potential of compounds people may already be consuming on a regular basis.
One particular polyphenol of interest is carnosol. Carnosol (Figure 1) is an ortho-diphenolic diterpene that contains an abietane carbon skeleton with hydroxyl groups at positions C-11 and C-12 and a lactone moiety across the B ring [13]. It is a naturally occurring polyphenol that is produced by the oxidative degradation of carnosic acid and is found in many herbs including rosemary (Rosmarinus officinalis) and sage (Salvia officinalis; Figure 1) [14][15][16]. The extracts of these plants have been demonstrated to have antioxidant, antimicrobial, antidiabetic, and anticancer properties with minimal toxicity [17][18][19][20]. Carnosol has been identified as a major component of these extracts which has led to research on the effects this individual compound has on various models of disease.
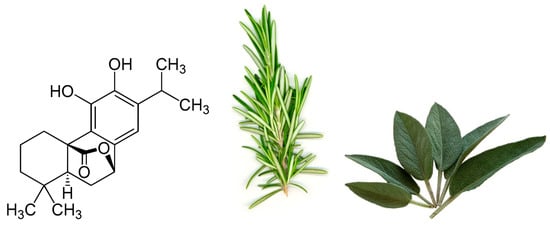
Figure 1. Chemical structure of carnosol found in rosemary and sage.
Carnosol has been proven to have potent antioxidant effects in cell-free, in vitro cell culture, and in vivo animal models. The accumulation of reactive oxygen species (ROS), formed through normal cellular functions as well as environmental exposure, can lead to the oxidation of DNA and proteins, which contributes to the development of diseases such as cancer [21][22]. Antioxidants can protect cells from ROS-induced damage by directly scavenging free radicals and/or activating endogenous enzymes and molecules that promote redox homeostasis [22][23]. In cell-free experiments, carnosol was able to inhibit lipid peroxidation, scavenge peroxyl radicals, protect anti-proteinase from hypochlorous acid-induced inactivation, scavenge hydroxyl radicals, and reduce cytochrome c, therefore confirming carnosol as a potent antioxidant [24]. Conversely, carnosol promoted bleomycin/iron-induced DNA damage indicating an ability to act as a pro-oxidant under certain conditions [24].
In vitro studies have demonstrated the nuclear factor erythroid derived 2-related factor 2 (Nrf2) to be involved in the cytoprotective effect of carnosol [25][26]. Treatment of HepG2 cells with 5 µM carnosol for 12 h exhibited a cytoprotective effect against both hydrogen peroxide and alcohol and resulted in a 160% increase in levels of glutathione (GSH), a tripeptide involved in the detoxification of chemical substances, with no adverse effect on cell viability [26]. Additionally, carnosol treatment led to increased levels of Nrf2 in the nucleus and Nrf2 knockdown via siRNA abolished the carnosol-induced increase of GSH synthesis enzymes [26]. Carnosol also supressed tumour necrosis factor alpha (TNF-α)-induced nuclear translocation of NF-κB, but this effect was abolished when carnosol treatment was performed alongside buthionine sulfoximine, a GSH synthesis blocker [26]. Another study found that carnosol (5–20 µM) treatment of microglia cells for 6 and 24 h was able to induce Nrf2 as well as heme oxygenase-1, an enzyme involved in counteracting oxidative stress, with minimal cytotoxicity [25]. These antioxidant properties of carnosol have also been confirmed in animal models where intraperitoneal injection of carnosol (100–400 mg/kg) in female Sprague–Dawley rats led to a 1.6- to 1.9-fold increase in glutathione-S-transferase (GST) activity and a 3.1- to 4.8-fold increase in NAD(P)H-quinone reductase (QR) activity, two enzymes involved in detoxification of chemical substances [27]. In summary, these studies show carnosol to exhibit antioxidant properties by directly scavenging free radicals as well as inducing cellular pathways that counteract oxidative stress. Studies provide evidence that many established cancer treatments such as paclitaxel increase ROS levels in cancer cells resulting in cytotoxic/cancer killing effects [28]. Therefore, more studies are required to investigate whether carnosol acts as a pro-oxidant or antioxidant in cancer cells in vitro and in clinical studies.
Although many studies provided evidence of the beneficial effects of carnosol against different diseases, only a limited number of studies have examined its bioavailability and pharmacokinetics [29][30][31][32]. Oral administration of 100 mg of rosemary extract (RE) enriched with carnosic acid to lean female Zucker rats produced a plasma carnosol concentration of 18.2 µM [31]. Furthermore, oral administration of RE resulted in carnosol being detected in tissues of the stomach, duodenum, jejunum, ileum, and liver, and only present in trace amounts in the brain [30][31]. These studies indicate that oral administration of rosemary extract, which contains carnosol, can result in blood carnosol levels in the micromolar range and significant levels in different tissues in the body. Many in vitro studies have shown that carnosol at micromolar levels can influence various biological functions and modify key signaling pathways [30][31][32]. Unfortunately, no animal studies exist where carnosol was administered orally or intraperitonially to animals followed by measurements of blood carnosol levels. In addition, no studies exist investigating the bioavailability and metabolism of carnosol in humans. Carnosol and polyphenols in general are hydrophobic and have low intestinal absorption rate. To bypass these absorption problem researchers are experimenting with encapsulation approaches but we have not found any such studies for carnosol as is the case for polyphenols such as resveratrol [33] and curcumin [34]. A study by Soler-Rivas et al. (2010) showed that carnosol present in a supercritical fluid extract of rosemary in sunflower oil (42 mg RE/g) is 62.59% bioaccessible to target tissues and increases to 87.85% by the addition of lecithin (37 mg/g) [29].
Cancer is characterized by the unregulated proliferation of cells, the inhibition of apoptosis, altered metabolism, tissue invasion and metastasis, and the dysregulation of cell signaling that ultimately leads to enhanced survival, growth, and tumour formation. The identification of compounds that can target these important cancer characteristics, without detrimentally affecting healthy normal cells/tissues is of the utmost urgency. Carnosol has been shown to possess antioxidant, anti-inflammatory, and antimicrobial properties and has been identified as a highly favored polyphenol for cancer prevention and treatment.
Treatment of breast cancer, prostate cancer, and skin cancer cells with carnosol significantly reduced cell viability, colony formation, cell proliferation and induced G2/M cell cycle arrest and apoptosis. Administration of carnosol in mice prevented the formation of DNA adducts and decreased the number of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary adenocarcinomas. Additionally, carnosol attenuated polycyclic aromatic hydrocarbon (PAH)-induced carcinogenesis in lung cancer cells via reduced DNA adduct formation. Colon cancer cells treated with carnosol had reduced viability, increased apoptosis, increased pro-apoptotic BAX expression and decreased anti-apoptotic Bcl-2 expression. Carnosol inhibited c-Met, induced apoptosis and prevented migration and colony formation in pancreatic cancer cells.
Oral administration of carnosol reduced tumour growth, serum prostate specific antigen levels and decreased androgen receptor (AR) and estrogen receptor-α (ER-α) protein expression in a xenograft model of prostate cancer. Treatment of differentiate glioblastoma cells with carnosol reduced cell viability and inhibited the processes that contribute to the aggressive and resistive nature of glioblastoma, including epithelial–mesenchymal transition (EMT) and cancer stem-like cell (CSC) formation. In addition, carnosol reduced TPA-induced ear inflammation, tumor formation and the average number of papillomata. The cellular effects of carnosol on the various subtypes of cancer are summarized in Figure 2.
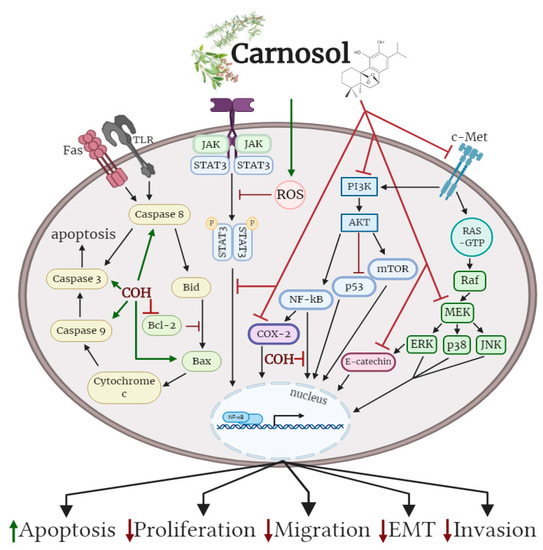
Figure 2. Effects of carnosol on cancer cell signaling molecules. Carnosol inhibited the phosphorylation/activation levels of ERK, p38, JNK, Akt, mTOR and COX-2. The nuclear translocation of NF-κB was prevented with carnosol, sequestering NF-κB in the cytosol. Pro-apoptotic caspase-3, -8, -9 and BAX protein levels were increased, while anti-apoptotic protein Bcl-2 was reduced with carnosol. The figure is based on the data of the studies [35][36][37][38][39][40][41][42][43][44][45][46] and created with BioRender.com. COH: carnosol.
Overall, these studies indicate that treatment with carnosol significantly attenuates key cancer characteristics, reducing cell viability, cell proliferation, colony formation, migration and promoting cancer cell apoptosis. However, more studies are required to fully understand the effects of carnosol in both cancerous and normal tissues. Notably, future in vitro studies should aim to expand our understanding of the effects of carnosol on cancers of the lung, colon, and pancreas as investigations involving cancer of these tissues are underrepresented in the literature. Additionally, more animal studies should be conducted including studies on the pharmacokinetics of carnosol to identify optimal dosage and routes of administration as well as xenograft studies to better understand the tumor reducing potential of carnosol to be used towards cancer treatment. Lastly, clinical studies are required to further explore the anticancer potential of carnosol in cancer patients.