1000/1000
Hot
Most Recent

The burgeoning field of nanotechnology aims to create and deploy nanoscale structures, devices, and systems with novel, size-dependent properties and functions. The nanotechnology revolution has sparked radically new technologies and strategies across all scientific disciplines, with nanotechnology now applied to virtually every area of research and development in the US and globally. NanoFlorida was founded to create a forum for scientific exchange, promote networking among nanoscientists, encourage collaborative research efforts across institutions, forge strong industry-academia partnerships in nanoscience, and showcase the contributions of students and trainees in nanotechnology fields. The 2019 NanoFlorida International Conference expanded this vision to emphasize national and international participation, with a focus on advances made in translating nanotechnology. This review highlights notable research in the areas of engineering especially in optics, photonics and plasmonics and electronics; biomedical devices, nano-biotechnology, nanotherapeutics including both experimental nanotherapies and nanovaccines; nano-diagnostics and -theranostics; nano-enabled drug discovery platforms; tissue engineering, bioprinting, and environmental nanotechnology, as well as challenges and directions for future research.
Nanotechnology is a dynamic revolutionary force that is exponentially impacting societies around the world. Nanotechnology and nanoscience aim to create, understand, and use nanoscale structures, devices, and systems having novel properties and functions. Through ground-breaking scientific and technological advancements, nanotechnology-based products are becoming fully integrated into our daily lives. The nanotechnology revolution embodies an interdisciplinary approach and is bringing scientists together from nearly every area of scientific study. This worldwide collaboration is significantly enhancing the quality of construction materials, machinery, automobiles, electronics, renewable energy, transportation, common appliances, consumer products, entertainment, agriculture, microscopy, scientific instrumentation, health care, diagnostic assays, and medicinal drug discovery. The US government investment in the National Nanotechnology Initiative was reported as nearly $29 billion in the 2020 budget supplement [1]. The anticipated returns on investments in terms of products (>2000 consumer products) globally has been estimated at >$3.3 trillion by the year 2025, and there were over 1900 U.S.-based companies conducting R&D, manufacturing, or product sales in nanotechnology in 2016. Attracting the upcoming generation of students to this burgeoning field and training of the work force in nanoscale technologies remains a major challenge and task for the future.
For the past twelve years a consortium of Florida universities has organized an annual conference on nanotechnology to address the demands of students and faculty conducting research on different aspects of nanotechnology. This conference also serves as a forum for academic and industrial researchers to share their recent discoveries, exchange new ideas, and develop professional relationships that strengthen the state’s nanotechnology research community. The annual NanoFlorida Conference provides a venue where expert nanoscientists from across the state of Florida, the US, and the world can come together with engineers, chemists, physicists, biologists, clinicians, and students to hear about the latest research in the field and discuss new directions and collaborations. The conference provides in-depth presentations and symposia for the experienced nanoscientist, as well as background and introductory talks for students and beginning researchers interested in applying nanotechnology in their own projects and for the clinician planning trials of new drug delivery systems, diagnostic devices, and therapeutic tools. The University of South Florida organized the 12th annual NanoFlorida Conference on November 15–17, 2019. The international conference featured three keynote speakers, sixteen plenary speakers, and nearly two hundred researchers who presented their work through oral and poster presentations.
Donald Martin’s group (University Grenoble Alpes, France) provided a historical introduction to the field of nanotechnology [2]. Their discussion outlined previous concepts introduced by Zsigmondy, Schmitt, Hibbs and Feynman that provide pillars upon which we can advance our ideas about nanostructured medical devices for implantation. To put a perspective on the modern era of nanotechnology, in an introductory plenary Lisa Friedersdorf (National Nanotechnology Coordination Office, Washington DC) spoke about “Nanotechnology Now and into the Future.” She gave an overview of how the launch of the U.S. National Nanotechnology Initiative brought much excitement to the area of nanotechnology, with the promise of lightweight materials, high-density data storage, and methods to detect cancer on the cellular level. Examples of ongoing nanotechnology research, current applications, and emerging areas that will influence the future were provided. A particular focus was placed on opportunities for students and faculty to participate in and build communities of interest related to nanotechnology research and development.
Ryan Gelfand (Tulane University, New Orleans, LA) described details of two revolutionary new biophysics techniques based on single protein optical trapping and provided potential examples of study for furthering our knowledge of how these processes can be applied to pharmaceutical research. Elucidating protein structure, function and behavior has been instrumental in increasing our understanding of human physiology. However, many of the tools developed for determining these properties have reached their limitations. Nanoaperture optical trapping (NAOT) is a nondestructive technique used to trap a single protein without any tethering, fluorophore labeling, or adhesion to a surface for many minutes, orders of magnitudes longer than other single molecule techniques. This freedom allows the protein to move in 3 dimensions without any steric hindrance, and so we can study them close to their native environments while still trapped. When a single molecule, such as a protein, is trapped, two things change about the transmitted signal through the nanoaperture: the amplitude and the variance. This effect is due to the polarizability of the protein and a modulation of this property can be observed and studied. Rate kinetics by nanoaperture optical trapping (RK-NAOT) uses the change in signal to determine the dynamics of a trapped protein and a small molecule with nanosecond resolution. When a protein binds to a small molecule, its shape changes resulting in a change in its polarizability which is evident in the collected signal. Furthermore, by using stimulated anti-stokes Raman scattering (STARS) spectroscopy, which is a two photon method, the vibrational modes of a protein can be elucidated and its structure can be potentially determined. These are just two of the many applications that NAOT can provide to the pharmaceutical research community.
Ratka Damnjanovic (University of South Florida, Tampa, FL) presented a novel proof of concept platform for spatially and temporally precise neural excitation, using hybrid electro-plasmonic stimulation technique in a whole-cell patch-clamp configuration to elicit electrical responses in primary trigeminal neurons. Neural stimulation prosthetics utilizing electrical stimulation have limited spatial resolution due to the spread of electrical currents to surrounding tissue. To facilitate specific point stimulation, various nanomaterial-assisted neural stimulation approaches have been reported [3][4][5][6][7][8][9][10][11][12][13][14] where different localized fields are activated. Infrared stimulation has been shown to excite neurons, but with a limitation of heating surrounding tissue [15][16]. In a prior study [17], visible green light and gold-nanoparticle-coated microelectrodes have been used for plasmonic modulation of SH-SY5Y neuroblastoma cells, but with limited success and detrimental effects on cell membranes with higher levels of pure optical stimulation. To address these limitations, herein, the first cellular study of the membrane effects produced by trigeminal neurons when stimulated with plasmonic and hybrid stimulation using visible light demonstrated that various combinations of sub-threshold levels of electrical and short-duration visible light (532 nm) pulses can successfully modulate neural firing patterns. The electrical stimulus amplitude required to evoke action potentials is significantly reduced (up to 40%) when a plasmonic stimulus (1–5 V, 1–5 ms pulse) is added, compared to electrical stimulation alone. Neuron cell survival and viability after hybrid stimulation is superior to that of pure optical stimulation (72% vs. 13%). The reduction of current required to trigger action potentials, and the finding that cells stay healthy after repeated exposure to hybrid stimulation, pave the way for a novel and more versatile generation of tunable neural stimulation systems. Specifically, these capabilities can play a key role in the development of prosthetic devices including cochlear implants that offer improved frequency modulation and specificity by more selective, focused and tunable activation of auditory neurons along the cochlear frequency axis.
Elnaz Zeynaloo (University of Miami, Miami, FL) presented a novel mediator-free, non-enzymatic electrochemical biosensor for direct glutamate monitoring, based on the immobilization of genetically engineered periplasmic glutamate binding protein (GluBP) onto gold nanoparticle (AuNP)-modified screen-printed carbon electrodes (SPCE). Glutamate is the brain’s most abundant free amino acid and the predominant excitatory transmitter. In addition to being the influencer of a variety of behaviors such as sensory perception, mood, and motor control, an excess of glutamate can cause excitotoxicity [18], which is a common pathological process in many neurologic disorders such as stroke [19], brain trauma [20], and Alzheimer’s disease [21]. Among various glutamate detection and monitoring technologies, electrochemical methods have shown great potential in point-of-care applications due to their high performance, easy miniaturization, low cost, and small sample volumes [22][23]. Enzymatic electrochemical biosensors are mostly used in glutamate detection [24][25][26]. However, enzyme-based electrochemical sensors still suffer from limitations, such as an indirect quantification of analytes, a requirement of redox mediators, and strong matrix interference (ascorbic acid) [27]. Cyclic voltammetry was performed to determine the glutamate and common interfering substrate concentration in a 50 mM sodium phosphate buffer solution (pH = 7.4). The results showed an excellent sensitivity with a detection limit of 0.1 µM and a linear range of 0.1 µM–1 µM of glutamate concentration. The sensor specificity was tested with a series of interfering substances, including amino acids (aspartate, glutamine, serine, and lysine) and a common matrix interference (ascorbic acid). The biosensor exhibited high selectivity toward glutamate over those substances, thus demonstrating its potential applications in biomedical and pharmaceutical analysis.
Ratnesh Lal (UCSD, San Diego, CA) discussed wireless electronic nano-biosensors for global health and security. Early and rapid diagnosis of biomarkers for diseases and transmissible infectious pathogens, including viruses, toxins, and bacteria inflicting large scale abnormalities and fatalities as well as plant diseases, are essential for effective global health and security. Existing approaches for the large scale assay of biomarkers use optical sensing, require amplification of the bio-sample, are time and resource consuming, require large laboratory setup, are non-portable, and lack sensitivity. On the other hand, nanodevices integrating nano-bio-probes and advanced nano-electronics with wireless capability as an array-biosensor platform on chips, with single molecule sensitivity and specificity, would overcome the abovementioned limitations of the current approaches. They can identify and monitor simultaneously an array of biomarkers of genetic defects, viruses, bacteria, toxins, and infectious airborne pathogens. The detected signals can be transmitted by Bluetooth to personal electronics, including smart phones, tablets, and computers. Lal presented an example of an integrated platform combining DNA nano-tweezers (a dynamic DNA nano-device) as a nucleic acid-sensing probe, electrical biosensors (using graphene FET), and an analytical wireless communication system [28]. The electrical signal resulting from the resistance changes triggered by the interaction between DNA nano-tweezer probes and defective DNA samples was recorded and transmitted remotely. Significantly, this new approach is label-free and does not require amplification of the probes to improve the detection sensitivity or an expensive immovable lab setup [29][30]. As such, implementation of this technology would allow cheaper, faster, implantable or wearable or hand-held, and portable point-of-care health status monitoring and accurate diagnoses of cancer, degenerative, genetic, and various communicable diseases as well as early detection of pathogens with potential for large population casualty.
Thomas Webster (Northeastern University, Boston, MA) discussed how nanotechnology can be used to increase tissue growth and decrease implant infection without using antibiotics but using sensors. His group has shown that nanofeatures, nano-modifications, nanoparticles, and most importantly nanosensors can reduce bacterial growth without using antibiotics. He summarized techniques and efforts to create nanosensors for a wide range of medical and tissue engineering applications, particularly those that have received FDA approval and are currently being implanted in humans [31]. Currently, there are a dozen or more ways to incorporate nanostructured features onto implants, changing many fundamental properties including surface area, surface chemistry, hydrophilicity, radio opacity, roughness, and catalytic properties that in turn affect surface energy and mechanical and electrical (conductivity) properties of the implanted materials such as catheters, stitches, or implants that are inserted for wound healing devices for the skin [32]. Interestingly, his lab developed a quantitative equation that can predict material protein and material cell behavior, so that one does not have to do this by trial and error [33]. Also, he discussed the use of micro- and nano- shot peening of stainless steel toward incorporating nanoscale surface features and nanoscale grain sizes which in turn increase osteoblast function and decrease bacterial growth, not killing mammalian cells but inhibiting bacteria cells, without drugs. Finally, he described the smart sensors in orthopedics that his group has been studying, which have three components: a component that senses what is going on in the body, a processor that processes the information and sends it to a computer or an iPhone, and a user interface where that sensor can then respond to the problem such as by increasing bone growth, decreasing infection, or decreasing scar tissue. He provided the example of a hip implant comprised of carbon nanotubes. These carbon nanotubes can measure the conductivity of the cell that attaches to the implant, and thus can determine whether it is a bone cell or a bacterial or an inflammatory cell. The hip implant also incorporates a wireless technology so that the sensor can then communicate the current status to a computer to inform the patient and the surgeon. Finally, a responding unit is incorporated made up of a polymer that can degrade under an applied electromagnetic field to release nanoparticles that could kill the bacteria, release bone growth factors that could increase bone growth, or release an anti-inflammatory agent if inflammation is the problem (Figure 1).
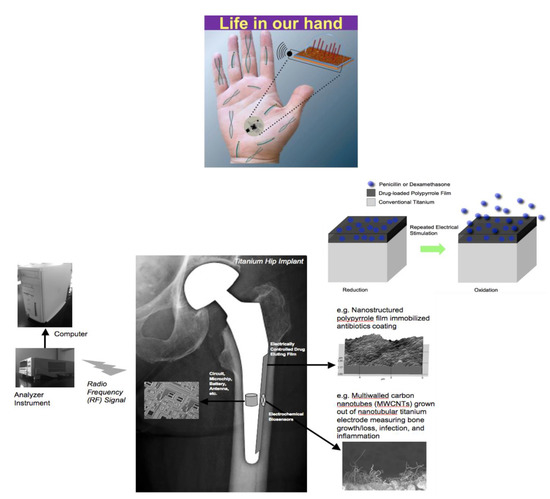
Figure 1. The SMARTHIPTM can detect cell presence and function next to a hip implant in real time and possesses on-demand release of nanoparticles, antibiotics, anti-inflammatory agents, and more to ensure implant success. Further, all information can be sent to a hand-held device to provide personalized medicine.
Donald Martin (University Grenoble Alpes, France) described the concept of “symbiotic nanostructured medical devices,” taking into account the fact that the implanted device is intended to have some communication of energy or materials with the body. If this is one-way communication from the device to the body, such as for a drug delivery, the challenge is to avoid the degradation or encapsulation of the device. However, if the implanted device is intended to restore body or organ function, then it needs to mimic the two-way (duplex) communication that is required for transplanted living organs or cells [34]. Examples of these implantable duplex communicating systems include biofuel cells or open-loop feedback devices where a molecule from the body is utilized by the duplex communicating system to produce a different material (e.g., molecule or energy) for use by the body. The challenges for such symbiotic systems are to be biocompatible and to maintain two-way transport communication of materials. Symbiotic devices extend the classical biocompatibility concept to include this functional requirement for continuous two-way (duplex) communication of materials with the body. It is likely that by utilizing such a biomimetic approach, scientists can be more efficient in mimicking what is happening at a molecular and cellular level to create a porous membrane that allows long-term exchanges of molecules between an implanted device and the body (Figure 2). Having this novel concept in mind will guide the research in a new field between medical implant and regenerative medicine to create actual symbiotic devices.
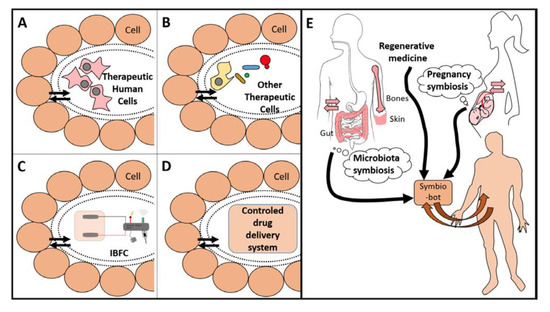
Figure 2. Examples of symbio-bots (A–D) that can be created in a bioinspired way (E). Each device is separated with a smart porous packaging that allows for duplex communication. Therapeutic cells (A and B) need a porous encapsulation that avoids an immune reaction and allows protection from both sides. They may be human cells, as MSC or specialized cells such as β-cell from Langherans islets (A), or other eukaryotic or prokaryotic cells (B). Panel (C) shows an IBFC linked to an electronic medical device. Panel (D) shows a generic device delivering a therapeutic molecule. Panel (E): Existing symbioses (i.e., microbiota or pregnancy) are a source of bio-inspiration to establish a duplex communication between the body and its implants. Regenerative medicine should embrace this concept of bioinspiration for better design and integration of implants, especially for future symbio-bots. (Reproduced with permission from [34].).
While many of the conference presentations highlight future directions in various fields of nanotechnology research, there remain several challenges. Despite the rapid development and escalating publications in this field, there remain a number of challenges in product development and commercialization.
One of the key issues is the health, safety and environmental aspects of some nanomaterials and their regulatory status. The responsible utilization and adoption of new technology will be impacted either favorably or unfavorably by the regulatory requirements for its oversight and further development. These regulatory requirements can either provide a platform which facilitates decision-making and responsible technology adoption or create unnecessary barriers to innovation and technology utilization. Regulations are aimed at identifying potential risks while avoiding unnecessary data generation, time delays, and increased costs. To this end, over the last two decades US agencies such as the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) have conducted evaluations of nanomaterials with respect to environmental sustainability across the life cycle and the challenges that remain in developing and applying scientific information to determine the safety of manufactured nanomaterials. In addition, international governance bodies are evolving to address the benefits of nanotechnologies while seeking to manage their potential risks to human health and the environment through a variety of voluntary, standard-setting, regulatory, statutory, and related governance platforms [35].
Particularly in relation to nanotherapeutics, a major issue has been that the Rule of Five that has been successfully used to predict the safety/toxicity of low molecular weight therapeutics developed for oral administration does not apply to nanomaterials. Indeed, the majority of nanomaterials in existence violate at least two or three of these rules [36]. Thus, manufacturers need to be vigilant and careful in the design and deployment of this useful class of novel materials.
Finally, there is a need to develop an integrated approach in order to balance appropriate regulatory oversight versus technology adoption, which was successfully used by DuPont [37] and comprised the development of a nano risk framework for responsible development involving a shared responsibility among all stakeholders including researchers, manufacturers, and regulating bodies. Then only can translational nanotechnology live up to its estimated return on investment.