
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Noushin Nasiri | + 3548 word(s) | 3548 | 2021-11-18 03:50:49 | | | |
| 2 | Catherine Yang | Meta information modification | 3548 | 2021-11-30 01:48:17 | | | | |
| 3 | Catherine Yang | Meta information modification | 3548 | 2021-11-30 01:48:53 | | |
Video Upload Options
Metal-organic frameworks (MOFs), a class of porous coordination polymers (PCPs), are crystalline frameworks with open porosity and are composed of metal nodes and organic linkers. Over the last two decades, numerous compounds have been synthesised by changing the metal ions and organic ligands to produce materials with exceptional properties, including large surface area (surface areas more than 1,000 m2g−1), adjustable pore size, and tunable functional groups. The manifold approaches for MOF synthesis, including the most versatile and widely used solvothermal methods, and recently realised green approaches, such as solvent-free mechanochemical routes, are making the process of preparing high-quality MOF-based materials easier and more environmentally friendly.
1. Introduction
In recent decades, the rapid growth of urban populations has resulted in new public health concerns and environmental pollution [1][2], and the fast monitoring of air- and water-borne contaminants using effective sensors has grown considerably in importance [1][3]. An effective sensor should interact with the target analyte selectively with high sensitivity and short response time [2][4][5][6]. In addition, the sensor should be cost-effective and demonstrate high reusability and reproducibility [2][7]. Sensors can be utilized to detect pollutants/analytes in aqueous (such as heavy metals, toxic organic compounds, and antibiotics) and gaseous form (such as volatile organic compounds, toxic gases, and greenhouse gases) [2][6][8][9]. The latter has a wide range of applications in food quality processes, industrial gases detection, and disease diagnosis, as well as indoor air-quality monitoring [8][10][11][12].
Several gas-sensing techniques have been developed including optical, capacitive, chemoresistive, and magnetic sensors [8][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33]. In optical sensing, material optical properties change upon adsorption of the target analyte onto the surface producing an optical signal such as a change in visible colour, refractive index, luminescence intensity, etc. [27][32]. Capacitive-based sensors are an attractive class of sensors, in which capacitance changes due to the change in the dielectric permittivity upon adsorbing the gas/vapour molecules are detected [28][29][30]. In chemoresistive detectors, electrical conductivity changes as a result of a reaction between the target gas and oxygen molecules adsorbed on the surface of the sensing material [4][21][33]. Generally, chemoresistive-based gas sensors provide a superior sensing response at room temperature compared to optical-based gas sensors. However, their slow response dynamic at low/room operating temperature and their lack of selectivity towards target gases hinder their real-world application [4][5][6].
In magnetic gas sensors, the magnetic properties of the sensing material are changed upon exposure to the target gas molecules; the change can be measured by, for example, application of the Hall effect, magnetization, spin orientation, ferromagnetic resonance, the magneto-optical Kerr effect, or the magnetostatic wave oscillation effect [8][13][14][15][17][18][19][20][21][22][23][24][25][26][31][34][35]. Sensing materials for gas detection can be classified into metal oxides [16][36][37], conductive and non-conductive polymers [38][39][40][41], carbon-based materials (e.g., graphene, carbon nanotubes, etc.) [42][43][44][45][46], noble metal-based structures [47][48][49][50], ionic liquids [51][52][53], metal-organic frameworks [8][54][55][56], and their composites [57][58][59]. Among these material groups, metal oxide-based sensors have been investigated extensively due to their strong and rapid response, low limit of detection (LOD), high reproducibility, simple and portable design, and low fabrication cost [60][61][62]. In recent years, the development of nanostructures and nanocomposites of metal oxide sensors has further improved device sensing characteristics. Despite these advantages and improvements, high operating temperature and inadequate gas selectivity have hindered substantial growth into new markets [61][63][64]. Polymers have been utilized in gas sensors as a sensing agent (mainly in a functionalized state) or immobilizing component to overcome some of these challenges [38][65]. Although significant progress in polymer sensors has been achieved over the last 20 years, these sensors encounter difficulties, including complex sensing mechanisms, poor selectivity towards target gases, a slow response dynamic, and significant matrix aging [22][38].
Among the unique properties of MOFs, the reversible and selective adsorption of guest molecules onto their large surface areas is of great importance for sensing applications [2][54]. In fact, a high concentration of target gases inside a highly porous structure boosts the sensitivity of the sensor and the control over functional groups and pore sizes of the framework enhances the selectivity of the detection process [54]. Furthermore, in contrast to carbon-based and metal oxide-based sensors, which require high working temperature, MOF-based sensors have shown promising performances at low/room temperatures, resulting in significant reductions in power consumption, ease of manufacture and broadened application areas [66][67][68][69]. The variation in MOFs’ physical and chemical properties following the adsorption of intended gas molecules has been exploited for the effective monitoring of environmental pollutants, indoor air quality, medical diagnosis and other areas of application [8][11][29][70][71].
2. Nitrogen Dioxide (NO2)
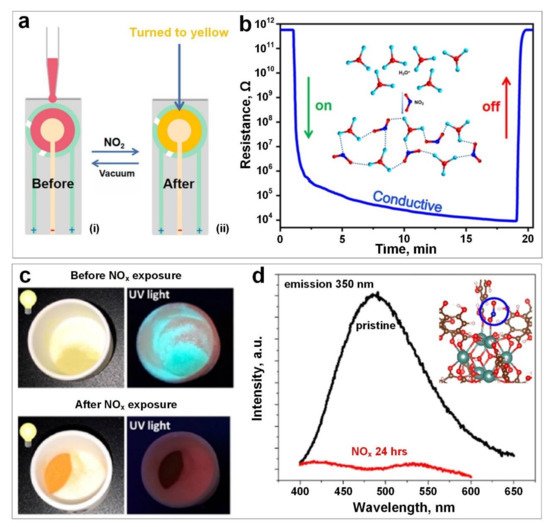
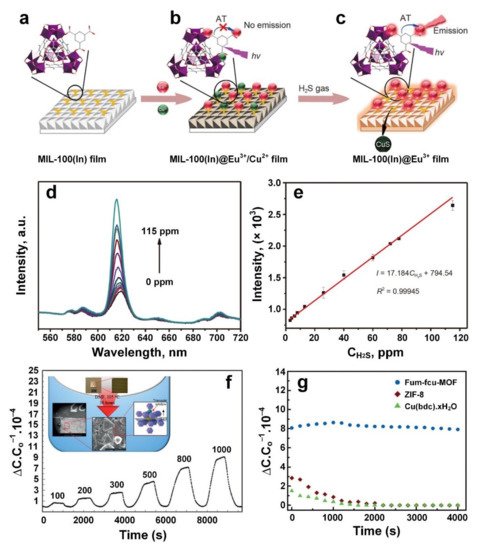
3. Hydrogen Sulphide (H2S)
4. Sulphur Dioxide (SO2)
Very recently, Zhang et al. [28] developed a capacitive-based sensing material using a relatively simple fabrication process ( Figure 3a) for the real-time monitoring of SO 2 gas molecules at room temperature. The sensing technology was introduced by using polyvinylidenefluoride (PVDF) nanofibers (with a diameter of 300–400 nm) coated with a thin layer of UiO-66-NH 2 MOF (200 nm in size) ( Figure 3b, inset) as a dielectric layer and carbon nanotubes (CNTs) as an electrode. The fabricated sensing material demonstrated a high sensitivity towards SO 2 in a large ppm range from 1 ppm to 150 ppm ( Figure 3b), high stability (over a testing period of 20 days) ( Figure 3c) and excellent bending flexibility (2000 bending cycles), with a short response time of 185 s for detecting a low concentration of SO 2 gas. The sensing mechanism was based on the change in the dielectric constant of UiO-66-NH 2 MOF layer due to the physical adsorption of SO 2 gas molecules in the MOF pores and voids. Interestingly, it demonstrated a faster response dynamic (both response and recovery time) after bending (compared to an unbended sample), which could be attributed to the shortened distance between the electrode and dielectric layer, resulting in a shorter transfer path for gas molecules to reach the dialectic layer, and consequently faster response dynamics.
Using a simple solvothermal technique, Chernikova et al. [84] deposited a layer of MFM-300 (a 3-periodic open framework composing of InO 4(OH) 2 octahedral chains bridged by tetradentate ligands (biphenyl-3,3’,5,5’-tetracarboxylic acid)) ( Figure 3d), on a silicon wafer featuring a capacitive interdigitated electrode functionalized with 11-Mercapto-1-undecanol, for the low concentration detection of SO 2 gas molecules. The sensing performance of the proposed porous nanostructured layer ( Figure 3e, inset) was investigated by monitoring the changes in capacitance upon exposure to a selection of different gas molecules including SO 2, CH 4, CO 2, NO 2 and H 2. The results showed outstanding detection sensitivity to SO 2 down to 75 ppb with a lower detection limit of 5 ppb and excellent selectivity towards SO 2 compared to other gases with slight cross-selectivity with CO 2 (four times less sensitive compared to SO 2).
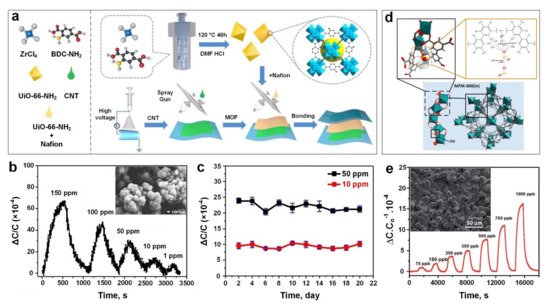
The effect of humidity level on sensing performance of the active film upon SO 2 exposure was investigated at 1000 and 350 ppb gas concentrations, and at relative humidity (RH) from 5% to 85%. In contrast to conventional gas sensors including metal oxide semiconductors, the sensing performance of the MFM-300-based gas sensor was enhanced significantly by increasing the RH up to 85%. This higher sensing response was attributed to the formation of additional hydrogen bonding between SO 2 gas molecules and adsorbed water molecules on the MOF’s surface, resulting in a higher capacitance change [85][86]. In addition, the MFM-300 layer demonstrated a higher sensing performance at lower operating temperature with an optimal operating temperature of 22 ᵒC, making it a promising material for highly sensitive, room temperature nanosensors for the ultra-low concentration detection of SO 2 gas molecules. This higher sensitivity could be attributed to a lower molecule diffusion rate and consequently, a higher analyte adsorption rate at a lower temperature [84].
In another approach, Ingle et al. [87] fabricated a flexible SO 2 gas sensor based on a crystalline nickel (II) benzenetricarboxylate metal-organic framework (Ni-MOF). In this device, the Ni-MOF composited with hydroxyl group (–OH) activated single-wall carbon nanotubes (SWNTs) and multi-wall carbon nanotubes (MWNTs), namely, Ni-MOF/–OH–SWNTs and Ni-MOF/–OH-MWNTs. Both CNT-modified Ni-MOF microdevices exhibited a discriminating response upon SO 2 exposure, which was contributed by the highly sensitive surface network of CNTs [88] providing favourable conditions for electron transportation [89]. The Ni-MOF/–OH-SWNTs sensor showed higher SO 2 sensing performance compared to the Ni-MOF/–OH-MWNTs at different SO 2 concentrations. This was due to holes being the majority charge careers in Ni-MOF/–OH-SWNTs [90] resulting in better interaction with electron donor analytes such as SO 2 gas molecules. However, a slow recovery speed was observed which could be attributed to the honeycomb structure of the CNTs as this plays a significant role in holding the gas molecules on the sensor’s surface for a longer time [88][91] prolonging the recovery dynamics of the device. A high sensing selectivity towards SO 2 molecules was also achieved using the MOF/CNT composite material compared to other gases, including NO 2, NH 3 and CO gases, at relatively high concentrations (≥10 ppm).
5. Carbon Dioxide (CO2)
Despite the modest greenhouse effect of CO 2 compared to methane (CH 4) and nitrous oxide (N 2O), which possess 25 and 298 times more global warming potential (GWP) than CO 2, respectively [92][93] , CO 2 is widely understood to be the major driver of climate change due to its dominant concentrations in the atmosphere (0.04 vol%) when compared to other types of greenhouse gas [94]. Sustained exposure to CO 2 gas indoors can cause inflammation and oxidative stress at a modest concentration level of 1000 ppm [95][96]. Thus, ongoing monitoring of indoor and outdoor CO 2 gas levels with reliable, portable and cost-effective sensing systems is highly desired in many industrial sectors.
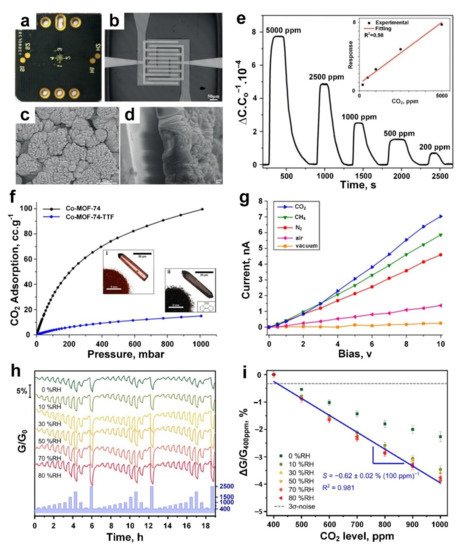
The sensing performance of the MOF film was investigated by measuring the capacitance change upon exposure to different gases including methane, benzene and CO 2, resulting in an outstanding sensing response of ~1 towards 200 ppm CO 2 vapour ( Figure 4e). This response is attributed to the interaction between unsaturated open metal sites of the Mg-MOF-74 crystallites (as an electron acceptor) and adsorbed CO 2 molecules acting as electron donors. A positive linear response was reported upon increasing the CO 2 gas concentration from 200 to 5000 ppm and attributed to the linear change in the dielectric constant of the Mg-MOF-74 layer over the gas adsorption on the surface ( Figure 4e, inset). However, no obvious response was detected for other gases, including methane, at similar concentrations. Post-synthesis modification of these MOF layers with ethylenediamine slightly increased their sensitivity towards CO 2. However, further investigations are required to reveal the key factors in the sensing performance of Mg-MOF-74 crystallites and their surface-analyte interaction with different gas molecules.
( a ) Photograph of Mg-MOF-74 film-based CO 2 capacitive gas sensor. ( b ) Optical image of Pt IDEs. ( c ,d ) SEM images of the top ( c ) and side ( d ) views of the as-grown Mg-MOF-74 film on IDEs. ( e ) The dynamic response of ethylenediamine-modified Mg-MOF-74 film against CO 2 at different concentrations, the response varied proportionally to the CO 2 concentration (inset). Reproduced with permission from [9] Wiley-VCH, 2019. ( f ) CO 2 adsorption isotherms of Co-MOF-74 (i) and Co-MOF-74-TTF (ii). ( g ) The I-V curves of Co-MOF-74-TTF under CO 2 and other atmospheric conditions. Reproduced with permission from [97] American Chemical Society, 2019. ( h ) The normalized dynamic response curves and, ( i ) response versus CO 2 gas at different concentrations for various humidity scenarios (0−80% RH). Reproduced with permission from [98] American Chemical Society, 2019.
To investigate the gas-sensing capability of Co-MOF-74-TTF, the I-V characteristics of the sensing material was measured after 24 h under vacuum, air, N 2, CH 4, and CO 2 atmospheres, respectively, at an applied voltage up to 10 V. The material demonstrated a higher sensing performance towards CO 2 gas molecules (current of 7 nA) compared to other gases including CH 4 (current of 6 nA) and N 2 (current of 4.5 nA) at 10 V ( Figure 4g). This higher sensing performance could be attributed to the strong interaction between open-metal Co-centres (acting as the Lewis acid) of MOF and CO 2 gas molecules (acting as the Lewis base/electron donor) resulting in the highest conductivity of the fabricated sensing material upon exposure to CO 2 gas ( Figure 4g), while a smaller increase in the conductivity was observed for weaker gas−MOF interactions (CO 2 > CH 4 > N 2). In fact, the permanent dipole moment, which is present in the cobalt atoms, could induce the polarization of molecules such as CH 4, resulting in the lower affinity of Co-MOF-74 toward CH 4 compared to CO 2 [99]. In addition, a significantly lower interaction was expected for N 2, as an inert gas, resulting in a lower sensing response towards N 2 gas molecules. Further investigation is required to resolve the poor selectivity and slow response dynamics (>500 min) of these Co-MOF-74-TTF for their real-world application as CO 2 gas sensors [97].
6. Summary and Outlook
High sensitivity and selectivity at low operating temperatures have been reported for state-of-the-art MOF-based sensors making them a promising candidate for various gas-sensing applications; however, MOF-based nanosensors for sub-ppb detection of gas molecules are still in the initial stages of development, with much room to improve the sensing performance of these MOF materials and many opportunities to enhance their LODs towards gas molecules.
High chemical, thermal, and photo stabilities have been achieved for some of the MOF-based sensing technologies, which determine their repeatability and long-term reusability. However, recent studies have indicated that some of the widely used MOFs for gas sensors undergo partial degradation upon exposure to moisture. Continued efforts are necessary for the design and development of robust MOF-based sensing materials to demonstrate long-term stability in different environments. Careful assessment of MOF sensors after long-term tests using analytical techniques sensitive to MOF degradation will enable researchers to improve their stability. The careful design of novel MOF structures (such as mixed metal ions, hydrophobic ligands, and interpenetration of frameworks), post-processing of current MOFs (such as metal/ligand exchange, hydrophobic surface modification, and thermal treatment), and compositing MOFs with, or encapsulating MOFs into, other materials (such as polymers, carbon nanotubes, graphenes and graphene oxide) are among the proposed techniques for improving the chemical stability of MOFs.
The inherently low electrical conductivity of most MOFs has severely limited their applications in gas and liquid sensors. However, the development of highly porous 2D conductive MOFs with tunable cavity size has facilitated the rise of MOF-based sensing technologies. In addition, the design of novel nanomaterials, including guest-MOF nanostructures where MOFs and guest molecules are combined, has shown excellent enhancement in the conductivity of MOF-based gas sensors.
The future is bright for the development of highly sensitive and selective MOF-based nanosensors for room temperature detection of low concentrations of gas molecules, with applications in many areas of technology, industry, or daily life, providing strong health, safety and security benefits to address many standing fundamental and technological challenges.
References
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285.
- Fang, X.; Zong, B.; Mao, S. Metal–Organic Framework-Based Sensors for Environmental Contaminant Sensing. Nano-Micro Lett. 2018, 10, 64.
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B 2020, 8, 3231–3248.
- Nasiri, N.; Clarke, C. Nanostructured Gas Sensors for Medical and Health Applications: Low to High Dimensional Materials. Biosensors 2019, 9, 43.
- Nasiri, N.; Clarke, C. Nanostructured Chemiresistive Gas Sensors for Medical Applications. Sensors 2019, 19, 462.
- Chen, X.; Leishman, M.; Bagnall, D.; Nasiri, N. Nanostructured Gas Sensors: From Air Quality and Environmental Monitoring to Healthcare and Medical Applications. Nanomaterials 2021, 11, 1927.
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210.
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401.
- Yuan, H.; Tao, J.; Li, N.; Karmakar, A.; Tang, C.; Cai, H.; Pennycook, S.J.; Singh, N.; Zhao, D. On-Chip Tailorability of Capacitive Gas Sensors Integrated with Metal–Organic Framework Films. Angew. Chem. 2019, 131, 14227–14232.
- Wang, H.; Lustig, W.P.; Li, J. Sensing and capture of toxic and hazardous gases and vapors by metal–organic frameworks. Chem. Soc. Rev. 2018, 47, 4729–4756.
- Rasheed, T.; Nabeel, F. Luminescent metal-organic frameworks as potential sensory materials for various environmental toxic agents. Coord. Chem. Rev. 2019, 401, 213065.
- Wenger, O.S. Vapochromism in Organometallic and Coordination Complexes: Chemical Sensors for Volatile Organic Compounds. Chem. Rev. 2013, 113, 3686–3733.
- Dolgopolova, E.A.; Rice, A.M.; Martin, C.R.; Shustova, N.B. Photochemistry and photophysics of MOFs: Steps towards MOF-based sensing enhancements. Chem. Soc. Rev. 2018, 47, 4710–4728.
- Yan, B. Lanthanide-Functionalized Metal–Organic Framework Hybrid Systems to Create Multiple Luminescent Centers for Chemical Sensing. Acc. Chem. Res. 2017, 50, 2789–2798.
- Wang, X.-D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761.
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 229, 206–217.
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840.
- Karmakar, A.; Samanta, P.; Desai, A.V.; Ghosh, S.K. Guest-Responsive Metal–Organic Frameworks as Scaffolds for Separation and Sensing Applications. Acc. Chem. Res. 2017, 50, 2457–2469.
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241.
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321.
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125.
- Reglero Ruiz, J.; Sanjuán, A.; Vallejos, S.; García, F.; García, J. Smart Polymers in Micro and Nano Sensory Devices. Chemosensors 2018, 6, 12.
- Ciprian, R.; Baratto, C.; Giglia, A.; Koshmak, K.; Vinai, G.; Donarelli, M.; Ferroni, M.; Campanini, M.; Comini, E.; Ponzoni, A.; et al. Magnetic gas sensing exploiting the magneto-optical Kerr effect on ZnO nanorods/Co layer system. RSC Adv. 2016, 6, 42517–42521.
- Matatagui, D.; Kolokoltsev, O.V.; Qureshi, N.; Mejía-Uriarte, E.V.; Saniger, J.M. A magnonic gas sensor based on magnetic nanoparticles. Nanoscale 2015, 7, 9607–9613.
- Zou, C.W.; Wang, J.; Liang, F.; Xie, W.; Shao, L.X.; Fu, D.J. Large-area aligned CuO nanowires arrays: Synthesis, anomalous ferromagnetic and CO gas sensing properties. Curr. Appl. Phys. 2012, 12, 1349–1354.
- Impeng, S.; Junkaew, A.; Maitarad, P.; Kungwan, N.; Zhang, D.; Shi, L.; Namuangruk, S. A MnN4 moiety embedded graphene as a magnetic gas sensor for CO detection: A first principle study. Appl. Surf. Sci. 2019, 473, 820–827.
- Zhu, C.; Gerald, R.E.; Huang, J. Metal-organic Framework Materials Coupled to Optical Fibers for Chemical Sensing: A Review. IEEE Sens. J. 2021, 1.
- Zhang, X.; Zhai, Z.; Wang, J.; Hao, X.; Sun, Y.; Yu, S.; Lin, X.; Qin, Y.; Li, C. Zr-MOF Combined with Nanofibers as an Efficient and Flexible Capacitive Sensor for Detecting SO2. ChemNanoMat 2021.
- Zhai, Z.; Zhang, X.; Hao, X.; Niu, B.; Li, C. Metal–Organic Frameworks Materials for Capacitive Gas Sensors. Adv. Mater. Technol. 2021, 2100127.
- Fernandez, E.; Saiz, P.G.; Peřinka, N.; Wuttke, S.; Fernández De Luis, R. Printed Capacitive Sensors Based on Ionic Liquid/Metal-Organic Framework Composites for Volatile Organic Compounds Detection. Adv. Funct. Mater. 2021, 31, 2010703.
- Srinivas, C.; Ranjith Kumar, E.; Tirupanyam, B.V.; Singh Meena, S.; Bhatt, P.; Prajapat, C.L.; Chandrasekhar Rao, T.V.; Sastry, D.L. Study of magnetic behavior in co-precipitated Ni–Zn ferrite nanoparticles and their potential use for gas sensor applications. J. Magn. Magn. Mater. 2020, 502, 166534.
- Sava Gallis, D.F.; Vogel, D.J.; Vincent, G.A.; Rimsza, J.M.; Nenoff, T.M. NOx Adsorption and Optical Detection in Rare Earth Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2019, 11, 43270–43277.
- Chen, H.; Bo, R.; Shrestha, A.; Xin, B.; Nasiri, N.; Zhou, J.; Di Bernardo, I.; Dodd, A.; Saunders, M.; Lipton-Duffin, J.; et al. NiO–ZnO Nanoheterojunction Networks for Room-Temperature Volatile Organic Compounds Sensing. Adv. Opt. Mater. 2018, 6, 1800677.
- Gerber, A.; Kopnov, G.; Karpovski, M. Hall effect spintronics for gas detection. Appl. Phys. Lett. 2017, 111, 143505.
- Alam, M.F.B.; Phan, D.-T.; Chung, G.-S. Palladium nanocubes decorated on a one-dimensional ZnO nanorods array for use as a hydrogen gas sensor. Mater. Lett. 2015, 156, 113–117.
- Yang, S.; Lei, G.; Xu, H.; Lan, Z.; Wang, Z.; Gu, H. Metal Oxide Based Heterojunctions for Gas Sensors: A Review. Nanomaterials 2021, 11, 1026.
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329.
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-based sensors: A review. Polym. Test. 2018, 67, 342–348.
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548.
- Ding, B.; Yamazaki, M.; Shiratori, S. Electrospun fibrous polyacrylic acid membrane-based gas sensors. Sens. Actuators B Chem. 2005, 106, 477–483.
- Barauskas, D.; Pelenis, D.; Vanagas, G.; Viržonis, D.; Baltrušaitis, J. Methylated Poly(ethylene)imine Modified Capacitive Micromachined Ultrasonic Transducer for Measurements of CO2 and SO2 in Their Mixtures. Sensors 2019, 19, 3236.
- Gargiulo, V.; Alfano, B.; Di Capua, R.; Alfé, M.; Vorokhta, M.; Polichetti, T.; Massera, E.; Miglietta, M.L.; Schiattarella, C.; Di Francia, G. Graphene-like layers as promising chemiresistive sensing material for detection of alcohols at low concentration. J. Appl. Phys. 2018, 123, 024503.
- Li, C.; Wang, Y.; Jiang, H.; Wang, X. Review—Intracellular Sensors Based on Carbonaceous Nanomaterials: A Review. J. Electrochem. Soc. 2020, 167, 037540.
- Wang, Y.; Yeow, J.T.W. A Review of Carbon Nanotubes-Based Gas Sensors. J. Sens. 2009, 2009, 493904.
- Zhang, T.; Mubeen, S.; Myung, N.V.; Deshusses, M.A. Recent progress in carbon nanotube-based gas sensors. Nanotechnology 2008, 19, 332001.
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45.
- Lee, J.M.; Park, J.-E.; Kim, S.; Kim, S.; Lee, E.; Kim, S.-J.; Lee, W. Ultra-sensitive hydrogen gas sensors based on Pd-decorated tin dioxide nanostructures: Room temperature operating sensors. Int. J. Hydrog. Energy 2010, 35, 12568–12573.
- Sharma, B.; Kim, J.-S. Graphene decorated Pd-Ag nanoparticles for H2 sensing. Int. J. Hydrog. Energy 2018, 43, 11397–11402.
- Zhang, S.; Yang, M.; Liang, K.; Turak, A.; Zhang, B.; Meng, D.; Wang, C.; Qu, F.; Cheng, W.; Yang, M. An acetone gas sensor based on nanosized Pt-loaded Fe2O3 nanocubes. Sens. Actuators B Chem. 2019, 290, 59–67.
- Barbosa, M.S.; Suman, P.H.; Kim, J.J.; Tuller, H.L.; Orlandi, M.O. Investigation of electronic and chemical sensitization effects promoted by Pt and Pd nanoparticles on single-crystalline SnO nanobelt-based gas sensors. Sens. Actuators B Chem. 2019, 301, 127055.
- Gębicki, J.; Kloskowski, A.; Chrzanowski, W.; Stepnowski, P.; Namiesnik, J. Application of Ionic Liquids in Amperometric Gas Sensors. Crit. Rev. Anal. Chem. 2016, 46, 122–138.
- Paul, A.; Muthukumar, S.; Prasad, S. Room-temperature ionic liquids for electrochemical application with special focus on gas sensors. J. Electrochem. Soc. 2019, 167, 037511.
- Zevenbergen, M.A.G.; Wouters, D.; Dam, V.-A.T.; Brongersma, S.H.; Crego-Calama, M. Electrochemical Sensing of Ethylene Employing a Thin Ionic-Liquid Layer. Anal. Chem. 2011, 83, 6300–6307.
- Li, Y.; Xiao, A.-S.; Zou, B.; Zhang, H.-X.; Yan, K.-L.; Lin, Y. Advances of metal–organic frameworks for gas sensing. Polyhedron 2018, 154, 83–97.
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. TrAC—Trends Anal. Chem. 2015, 73, 39–53.
- Hu, M.-L.; Razavi, S.A.A.; Piroozzadeh, M.; Morsali, A. Sensing organic analytes by metal–organic frameworks: A new way of considering the topic. Inorg. Chem. Front. 2020, 7, 1598–1632.
- Abideen, Z.U.; Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Electrospun Metal Oxide Composite Nanofibers Gas Sensors: A Review. J. Korean Ceram. Soc. 2017, 54, 366–379.
- Wang, C.; Wang, Y.; Yang, Z.; Hu, N. Review of recent progress on graphene-based composite gas sensors. Ceram. Int. 2021, 47, 16367–16384.
- Love, C.; Nazemi, H.; El-Masri, E.; Ambrose, K.; Freund, M.S.; Emadi, A. A Review on Advanced Sensing Materials for Agricultural Gas Sensors. Sensors 2021, 21, 3423.
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141.
- Wang, J.; Shen, H.; Xia, Y.; Komarneni, S. Light-activated room-temperature gas sensors based on metal oxide nanostructures: A review on recent advances. Ceram. Int. 2021, 47, 7353–7368.
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured Metal Oxide-Based Acetone Gas Sensors: A Review. Sensors 2020, 20, 3096.
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106.
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001.
- Adhikari, B.; Majumdar, S. Polymers in sensor applications. Prog. Polym. Sci. 2004, 29, 699–766.
- Song, L.-F.; Jiang, C.-H.; Jiao, C.-L.; Zhang, J.; Sun, L.-X.; Xu, F.; You, W.-S.; Wang, Z.-G.; Zhao, J.-J. Two New Metal−Organic Frameworks with Mixed Ligands of Carboxylate and Bipyridine: Synthesis, Crystal Structure, and Sensing for Methanol. Cryst. Growth Des. 2010, 10, 5020–5023.
- Achmann, S.; Hagen, G.; Kita, J.; Malkowsky, I.; Kiener, C.; Moos, R. Metal-Organic Frameworks for Sensing Applications in the Gas Phase. Sensors 2009, 9, 1574–1589.
- Yao, M.-S.; Lv, X.-J.; Fu, Z.-H.; Li, W.-H.; Deng, W.-H.; Wu, G.-D.; Xu, G. Layer-by-Layer Assembled Conductive Metal-Organic Framework Nanofilms for Room-Temperature Chemiresistive Sensing. Angew. Chem. 2017, 129, 16737–16741.
- Arul, C.; Moulaee, K.; Donato, N.; Iannazzo, D.; Lavanya, N.; Neri, G.; Sekar, C. Temperature modulated Cu-MOF based gas sensor with dual selectivity to acetone and NO2 at low operating temperatures. Sens. Actuators B Chem. 2021, 329.
- Koo, W.T.; Jang, J.S.; Kim, I.D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963.
- Lai, C.; Wang, Z.; Qin, L.; Fu, Y.; Li, B.; Zhang, M.; Liu, S.; Li, L.; Yi, H.; Liu, X.; et al. Metal-organic frameworks as burgeoning materials for the capture and sensing of indoor VOCs and radon gases. Coord. Chem. Rev. 2021, 427, 213565.
- Gaba, A.; Felicia, S. Reduction of Air Pollution by Combustion Processes. In The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources; InTech: London, UK, 2011; pp. 119–142.
- WHO. Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005; WHO: Geneva, Switzerland, 2006.
- Chen, T.-M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor Air Pollution: Nitrogen Dioxide, Sulfur Dioxide, and Carbon Monoxide Health Effects. Am. J. Med. Sci. 2007, 333, 249–256.
- Gamonal, A.; Sun, C.; Mariano, A.L.; Fernandez-Bartolome, E.; Guerrero-Sanvicente, E.; Vlaisavljevich, B.; Castells-Gil, J.; Marti-Gastaldo, C.; Poloni, R.; Wannemacher, R.; et al. Divergent Adsorption-Dependent Luminescence of Amino-Functionalized Lanthanide Metal–Organic Frameworks for Highly Sensitive NO2 Sensors. J. Phys. Chem. Lett. 2020, 11, 3362–3368.
- Zhang, J.; Hu, E.; Liu, F.; Li, H.; Xia, T. Growth of robust metal–organic framework films by spontaneous oxidation of a metal substrate for NO2 sensing. Mater. Chem. Front. 2021.
- Moscoso, F.G.; Almeida, J.; Sousaraei, A.; Lopes-Costa, T.; Silva, A.M.G.; Cabanillas-Gonzalez, J.; Cunha-Silva, L.; Pedrosa, J.M. Luminescent MOF crystals embedded in PMMA/PDMS transparent films as effective NO2 gas sensors. Mol. Syst. Des. Eng. 2020, 5, 1048–1056.
- Kim, J.-O.; Koo, W.-T.; Kim, H.; Park, C.; Lee, T.; Hutomo, C.A.; Choi, S.Q.; Kim, D.S.; Kim, I.-D.; Park, S. Large-area synthesis of nanoscopic catalyst-decorated conductive MOF film using microfluidic-based solution shearing. Nat. Commun. 2021, 12.
- Ma, Y.-X.; Gao, B.; Li, Y.; Wei, W.; Zhao, Y.; Ma, J.-F. Macrocycle-Based Metal–Organic Frameworks with NO2-Driven On/Off Switch of Conductivity. ACS Appl. Mater. Interfaces 2021, 13, 27066–27073.
- Meng, C.; Cui, X.; Qi, S.; Zhang, J.; Kang, J.; Zhou, H. Lung inflation with hydrogen sulfide during the warm ischemia phase ameliorates injury in rat donor lungs via metabolic inhibition after cardiac death. Surgery 2017, 161, 1287–1298.
- Zhou, X.; Lin, X.; Yang, S.; Zhu, S.; Chen, X.; Dong, B.; Bai, X.; Wen, X.; Geyu, L.; Song, H. Highly dispersed Metal–Organic-Framework-Derived Pt nanoparticles on three-dimensional macroporous ZnO for trace-level H2S sensing. Sens. Actuators B Chem. 2020, 309, 127802.
- Zhang, J.; Liu, F.; Gan, J.; Cui, Y.; Li, B.; Yang, Y.; Qian, G. Metal-organic framework film for fluorescence turn-on H2S gas sensing and anti-counterfeiting patterns. Sci. China Mater. 2019, 62, 1445–1453.
- Yassine, O.; Shekhah, O.; Assen, A.H.; Belmabkhout, Y.; Salama, K.N.; Eddaoudi, M. H2S Sensors: Fumarate-Based fcu-MOF Thin Film Grown on a Capacitive Interdigitated Electrode. Angew. Chem. 2016, 128, 16111–16115.
- Chernikova, V.; Yassine, O.; Shekhah, O.; Eddaoudi, M.; Salama, K.N. Highly sensitive and selective SO2 MOF sensor: The integration of MFM-300 MOF as a sensitive layer on a capacitive interdigitated electrode. J. Mater. Chem. A 2018, 6, 5550–5554.
- Assen, A.H.; Yassine, O.; Shekhah, O.; Eddaoudi, M.; Salama, K.N. MOFs for the Sensitive Detection of Ammonia: Deployment of fcu-MOF Thin Films as Effective Chemical Capacitive Sensors. ACS Sens. 2017, 2, 1294–1301.
- Sapsanis, C.; Omran, H.; Chernikova, V.; Shekhah, O.; Belmabkhout, Y.; Buttner, U.; Eddaoudi, M.; Salama, K. Insights on Capacitive Interdigitated Electrodes Coated with MOF Thin Films: Humidity and VOCs Sensing as a Case Study. Sensors 2015, 15, 18153–18166.
- Ingle, N.; Sayyad, P.; Deshmukh, M.; Bodkhe, G.; Mahadik, M.; Al-Gahouari, T.; Shirsat, S.; Shirsat, M.D. A chemiresistive gas sensor for sensitive detection of SO2 employing Ni-MOF modified –OH-SWNTs and –OH-MWNTs. Appl. Phys. A 2021, 127.
- Seesaard, T.; Kerdcharoen, T.; Wongchoosuk, C. Hybrid materials with carbon nanotubes for gas sensing. In Semiconductor Gas Sensors, 2nd ed.; Jaaniso, R., Tan, O.K., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 185–222.
- Ma, P.-C.; Liu, M.-Y.; Zhang, H.; Wang, S.-Q.; Wang, R.; Wang, K.; Wong, Y.-K.; Tang, B.-Z.; Hong, S.-H.; Paik, K.-W.; et al. Enhanced Electrical Conductivity of Nanocomposites Containing Hybrid Fillers of Carbon Nanotubes and Carbon Black. ACS Appl. Mater. Interfaces 2009, 1, 1090–1096.
- Ingle, N.; Mane, S.; Sayyad, P.; Bodkhe, G.; Al-Gahouari, T.; Mahadik, M.; Shirsat, S.; Shirsat, M.D. Sulfur Dioxide (SO2) Detection Using Composite of Nickel Benzene Carboxylic (Ni3BTC2) and OH-Functionalized Single Walled Carbon Nanotubes (OH-SWNTs). Front. Mater. Sci. 2020, 7, 93.
- Mao, S.; Lu, G.; Chen, J. Nanocarbon-based gas sensors: Progress and challenges. J. Mater. Chem. A 2014, 2, 5573.
- Boucher, O.; Friedlingstein, P.; Collins, B.; Shine, K.P. The indirect global warming potential and global temperature change potential due to methane oxidation. Environ. Res. Lett. 2009, 4, 044007.
- Hou, H.; Peng, S.; Xu, J.; Yang, S.; Mao, Z. Seasonal variations of CH4 and N2O emissions in response to water management of paddy fields located in Southeast China. Chemosphere 2012, 89, 884–892.
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emission. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 22 August 2021).
- Ramalho, O.; Wyart, G.; Mandin, C.; Blondeau, P.; Cabanes, P.-A.; Leclerc, N.; Mullot, J.-U.; Boulanger, G.; Redaelli, M. Association of carbon dioxide with indoor air pollutants and exceedance of health guideline values. Build Environ. 2015, 93, 115–124.
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701.
- Strauss, I.; Mundstock, A.; Treger, M.; Lange, K.; Hwang, S.; Chmelik, C.; Rusch, P.; Bigall, N.C.; Pichler, T.; Shiozawa, H.; et al. Metal–Organic Framework Co-MOF-74-Based Host–Guest Composites for Resistive Gas Sensing. ACS Appl. Mater. Interfaces 2019, 11, 14175–14181.
- Stassen, I.; Dou, J.-H.; Hendon, C.; Dincă, M. Chemiresistive Sensing of Ambient CO2 by an Autogenously Hydrated Cu3(hexaiminobenzene)2 Framework. ACS Cent. Sci. 2019, 5, 1425–1431.
- García, E.J.; Mowat, J.P.S.; Wright, P.A.; Pérez-Pellitero, J.; Jallut, C.; Pirngruber, G.D. Role of Structure and Chemistry in Controlling Separations of CO2/CH4 and CO2/CH4/CO Mixtures over Honeycomb MOFs with Coordinatively Unsaturated Metal Sites. J. Phys. Chem. C 2012, 116, 26636–26648.




