
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Radim Vrzal | + 1709 word(s) | 1709 | 2021-11-15 10:20:22 | | | |
| 2 | Amina Yu | + 41 word(s) | 1750 | 2021-11-16 04:41:11 | | | | |
| 3 | Amina Yu | Meta information modification | 1750 | 2021-11-17 10:20:27 | | |
Video Upload Options
Cytochrome P450 2A13 (CYP2A13) can be found primarily outside the gastrointestinal tract. Possibly that was the reason, why it received less attention than CYP2A6, which forms approximately 3.5–14% of all human CYPs in the liver and was investigated in more detail towards the drug metabolism. However, CYP2A13 plays a crutial part in metabolism of smoke-related carcinogens and in some lung cancers.
1. Introduction
Xenobiotic-metabolizing enzymes (XMEs) play a crucial role in the detoxification of foreign compounds. The most abundant subgroup is the cytochrome P450 superfamily (CYP; 1.14.X.X), a heme that contains enzymes that participate in phase I of biotransformation. Usually, they are bound to the membrane of the endoplasmic reticulum with the C-terminus and using NADPH generated by cytochrome P450 reductase (CYPOR; 1.6.2.4). They incorporate one atom of molecular oxygen into the xenobiotic molecule, and the second one is combined with hydrogen to form water. Due to this property, they are called monooxygenases or mixed function oxidases. The superfamily (CYP) is divided into families (e.g., CYP2) by at least 40% amino-acid sequence homology, and further into subfamilies (e.g., CYP2A), where members must share at least a 55% amino acid identity. Each individual member within the subfamily is further marked with a number (e.g., CYP2A6, CYP2A13). Usually, these are located on the same chromosome, and it is believed that they were created throughout the evolution by gene duplication of the whole CYP superfamily [1][2][3]). These enzymes are responsible for the metabolism of xeno-biotics (e.g., drugs, environmental pollutants), as well as endo-biotics (e.g., fatty acids, steroids) [4][5][6].
The CYP2A subfamily consists of three complete genes so far: CYP2A6, CYP2A7, and CYP2A13, first identified in 1995 [7]. Due to the primary presence of CYP2A13 outside the gastrointestinal tract, it receives less attention than CYP2A6, which forms approximately 3.5–14% of all human CYPs in the liver [8]. Therefore, it was investigated in more detail towards the drug metabolism.
Interestingly, CYP2A13 is abundantly expressed in lung tissue and is considered a significant player in the tobacco-induced lung cancer process. One of the key components of cigarette smoke is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a tobacco-specific nitrosamine ketone, the metabolism of which is mediated by CYP2A13. Since this compound was labeled as a carcinogen by the International Agency for Research on Cancer (IARC) in 2012, it is inseparably connected with CYP2A13.
Due to CYP2A13 being less investigated than CYP2A6 (approximately 10 times fewer hits at PUBMED in September 2021), the significant role in the biotransformation of tobacco carcinogens, and possibly the key role in the pathology of tobacco-induced lung cancer, this mini-review summarizes basic knowledge about CYP2A13.
2. Tissue Distribution
The effort to detect CYP2A13 in different human tissues started with the detection of a transcript. The likely reason for this approach was probably the lower detection limit and better control of factors (primer sequence, magnesium concentration) needed for transcript detection. Protein detection was always (and often still is) more challenging; in particular, when there is more than a 93% amino acid sequence similarity with CYP2A6 and the quality of specific antibodies is subjected to an immune system of a given organism, where antibodies are produced.
Thus, the initial search detected the CYP2A13 transcript in the liver and various extrahepatic tissues (e.g., lung, trachea, brain, mammary gland, prostate, testes) but not in the heart, kidney, bone marrow, colon, small intestine, spleen, stomach, thymus, or skeletal muscle [9]. In this study, the liver tissue contained the least CYP2A13 mRNA, next to the lung < trachea < nasal mucosa. Confirmation of CYP2A13 high expression in olfactory mucosa (OM) was performed in human nasal microsomes from human fetal tissues at different gestational ages (G91-G125) [10]. This study suggested that human fetal OM may be a preferred target tissue for the toxicity of maternally-derived chemical compounds that are activated by the CYP2A enzymes, and may have a greater impact on behavior, growth, and development than in adults.
Although transcript detection is relatively straightforward, protein detection caused more confusion. As an example, it may serve the study, where the presence of the CYP2A13 protein was detected in only 12% of 116 human lung microsomal samples by high-resolution immunoblotting followed by immunopurification with an anti-CYP2A5 antibody (mouse ortholog of CYP2A6) [11]. Since the level of CYP2A13, but not CYP2A6, was correlated with lung microsomal NNK metabolic activity, it was speculated that people with relatively high levels of CYP2A13 expression are likely to have an increased risk of developing smoking-related lung cancer.
A better level of the specific tissue distribution of CYP2A13 was reached with a peptide-specific antibody against human CYP2A13 that did not cross-react with CYP2A6 or CYP2A5 [12]. It was found that a high level of CYP2A13 protein expression can be found in the epithelial cells of the human bronchus and trachea, but a rare distribution in the alveolar cells. Interestingly, there was little expression of the CYP2A13 protein in different types of human lung carcinomas. This suggests that most smoking-related human lung cancers are bronchogenic and that the regulation of CYP2A13 expression is not altered in lung cancer cells [12].
3. Genetic Polymorphisms
Therefore, it is of interest to monitor whether there is a correlation between certain CYP2A13 polymorphisms and the incidence of any type of cancer. Interestingly, a significant genotype effect was found for the CYP2A13 *3 allele and 10 cigarettes smoked per day group within a cohort of Spanish smokers [13]. In contrast, no association between CYP2A13 polymorphisms and lung cancer was found in the Japanese population [14]. However, the risk of bladder cancer was revealed recently for CYP2A13 *1/*2 genotypes in Japanese smokers [15].
Another type of association of CYP2A13 polymorphism and head and neck cancer was observed in a cohort of North Indians [16]. In a comparison of a group of 203 head and neck cancer patients, next to the 201 healthy controls, two novel polymorphisms of CYP2A13 (T478C and T494C) were detected that were associated with a significantly reduced risk of cancer. On the contrary, the C578T mutant allele of CYP2A13 was found only in cancer patients.
The reduction in lung adenocarcinoma has been associated with CYP2A13 *2 genetic polymorphism [17] given by C→T transition, leading to Arg257Cys substitution and reduced activity of CYP2A13 towards NNK. A stratification analysis demonstrated that the reduced risk of lung adenocarcinoma was related to the CYP2A13 variant and was limited to smokers, especially light smokers, but not non-smokers or heavy smokers [17].
A variant allele of CYP2A13 ( CYP2A13 *2) was previously found to be associated with a lower incidence of lung adenocarcinoma in smokers and was associated with a lower level of expression compared to the CYP2A13 *1 allele [18]. Furthermore, a 26 nucleotide deletion was discovered, which caused a decrease in CYP2A13 promoter activity in the human lung cell line A549. These findings suggested that the reported association of the CYP2A13 *2 allele with a lower incidence of lung adenocarcinoma in smokers may be at least partially explained by a decrease in CYP2A13 function.
4. Transcription Regulation
Some aspects of CYP2A13 expression regulation can be deduced from cancer cells. In the study, where CYP2A13 expression was examined in non-small cell lung cancer (NSCLC) tissues, researchers observed the downregulation of CYP2A13 in lung adenocarcinoma [19]. This observation was confirmed in another study [20]. On the contrary, a marked increase in CYP2A13 in NSCLC was described in 2010 using immunohistochemical analysis [21]. However, it was suggested that the high expression of CYP2A13 might be associated with tumor development and progression in non-small cell lung carcinomas.
The possible regulation or implication of the estrogen receptor (ER) in CYP2A13 expression can be speculated from the case control study, where a nonsignificant increase in lung cancer risk was observed in the group of women carriers of the minor allele of CYP2A13 SNP rs1709084 (13103A>G), in which, the effect was further modified by smoking [22]. In particular, for light and never-smokers (0–10 pack-year), this minor genotype was associated with an increased risk of lung cancer. Furthermore, the three-way interaction between gender, smoking, and the genotype of CYP2A13 was statistically significant [22]. It was concluded that this CYP2A13 genotype may contribute to individual susceptibility to early-onset lung cancer in women. However, so far, that would investigate or identify the relationship between ER and/or FOXA2 and CYP2A13 expression, there are some indications that such a relationship could exist. One such indicia comes from studies that tried to decipher sexual dimorphism for hepatocellular carcinoma (HCC), where a significantly lower incidence in women exists [23]. Since this phenomenon is the same for rodents as it is for humans, it was demonstrated in female mouse liver that Foxa2 is complexed with ERalpha [24]. However, since these observations were made in mice and in liver, not in humans and in the lungs or bladder, the true role of ER in CYP2A13 expression represents a further research direction. Similarly, the very same role of the androgen receptor (AR) should also be investigated, since a direct interaction of AR with Foxa2 was identified in the mouse epididymal epithelial cell line DC2 [25]. Therefore, sexual hormones can affect the expression of CYP2A13, but this must be verified experimentally.
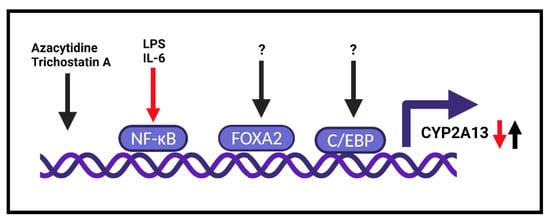
However, the role of inflammation can be tricky, since AFG1, which can induce chronic lung inflammation, increased CYP2A13 expression together with DNA damage in AFG1-induced inflamed lung tissues [26]. Furthermore, mice treated with the soluble TNFα receptor and AFG1 demonstrated that TNFα inhibited AFG1-induced chronic lung inflammation in vivo and reversed CYP2A13 expression and DNA damage in primary mouse alveolar type II (AT-II) cells. However, by using human AT-II-like cells (A549), the authors found an enhancement of AFG1-induced DNA damage with TNFα. Blocking the NF-kB pathway with siRNA resulted in the inhibition of TNFα-induced CYP2A13 [26].
Interestingly, by using epigenetic agents (azacytidine—DNA demethylation agent and trichostatin A—histone deacetylation inhibitor), it was found that CYP2A13 expression is induced in NCI-H441 human lung cancer cells [27] ( Figure 1 ). This observation was confirmed 6 years later, and the presence of LPS or IL-6 suppressed the induction of CYP2A13 mRNA by epigenetic agents [28].
References
- Omura, T. Structural diversity of cytochrome P450 enzyme system. J. Biochem. 2010, 147, 297–306.
- Tralau, T.; Luch, A. The evolution of our understanding of endo-xenobiotic crosstalk and cytochrome P450 regulation and the therapeutic implications. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1541–1554.
- Podust, L.M.; Sherman, D.H. Diversity of P450 enzymes in the biosynthesis of natural products. Nat. Prod. Rep. 2012, 29, 1251–1266.
- Nebert, D.W.; Dalton, T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960.
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120431.
- Lewis, D.F. 57 varieties: The human cytochromes P450. Pharmacogenomics 2004, 5, 305–318.
- Fernandez-Salguero, P.; Hoffman, S.M.; Cholerton, S.; Mohrenweiser, H.; Raunio, H.; Rautio, A.; Pelkonen, O.; Huang, J.D.; Evans, W.E.; Idle, J.R.; et al. A genetic polymorphism in coumarin 7-hydroxylation: Sequence of the human CYP2A genes and identification of variant CYP2A6 alleles. Am. J. Hum. Genet. 1995, 57, 651–660.
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141.
- Su, T.; Bao, Z.; Zhang, Q.Y.; Smith, T.J.; Hong, J.Y.; Ding, X. Human cytochrome P450 CYP2A13: Predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000, 60, 5074–5079.
- Gu, J.; Su, T.; Chen, Y.; Zhang, Q.Y.; Ding, X. Expression of biotransformation enzymes in human fetal olfactory mucosa: Potential roles in developmental toxicity. Toxicol. Appl. Pharmacol. 2000, 165, 158–162.
- Zhang, X.; D’Agostino, J.; Wu, H.; Zhang, Q.Y.; von Weymarn, L.; Murphy, S.E.; Ding, X. CYP2A13: Variable expression and role in human lung microsomal metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. J. Pharmacol. Exp. Ther. 2007, 323, 570–578.
- Zhu, L.R.; Thomas, P.E.; Lu, G.; Reuhl, K.R.; Yang, G.Y.; Wang, L.D.; Wang, S.L.; Yang, C.S.; He, X.Y.; Hong, J.Y. CYP2A13 in human respiratory tissues and lung cancers: An immunohistochemical study with a new peptide-specific antibody. Drug Metab. Dispos. 2006, 34, 1672–1676.
- Verde, Z.; Santiago, C.; Rodriguez Gonzalez-Moro, J.M.; de Lucas Ramos, P.; Lopez Martin, S.; Bandres, F.; Lucia, A.; Gomez-Gallego, F. ‘Smoking genes’: A genetic association study. PLoS ONE 2011, 6, e26668.
- Tamaki, Y.; Arai, T.; Sugimura, H.; Sasaki, T.; Honda, M.; Muroi, Y.; Matsubara, Y.; Kanno, S.; Ishikawa, M.; Hirasawa, N.; et al. Association between cancer risk and drug-metabolizing enzyme gene (CYP2A6, CYP2A13, CYP4B1, SULT1A1, GSTM1, and GSTT1) polymorphisms in cases of lung cancer in Japan. Drug Metab. Pharmacokinet. 2011, 26, 516–522.
- Kumondai, M.; Hosono, H.; Orikasa, K.; Arai, Y.; Arai, T.; Sugimura, H.; Ozono, S.; Sugiyama, T.; Takayama, T.; Sasaki, T.; et al. CYP2A13 genetic polymorphisms in relation to the risk of bladder cancer in Japanese smokers. Biol. Pharm. Bull. 2016, 39, 1683–1686.
- Sharma, R.; Ahuja, M.; Panda, N.; Khullar, M. Polymorphisms in CYP2A13 and UGT1A7 genes and head and neck cancer susceptibility in North Indians. Oral Dis. 2010, 16, 760–768.
- Wang, H.; Tan, W.; Hao, B.; Miao, X.; Zhou, G.; He, F.; Lin, D. Substantial reduction in risk of lung adenocarcinoma associated with genetic polymorphism in CYP2A13, the most active cytochrome P450 for the metabolic activation of tobacco-specific carcinogen NNK. Cancer Res. 2003, 63, 8057–8061.
- D’Agostino, J.; Zhang, X.; Wu, H.; Ling, G.; Wang, S.; Zhang, Q.Y.; Liu, F.; Ding, X. Characterization of CYP2A13*2, a variant cytochrome P450 allele previously found to be associated with decreased incidences of lung adenocarcinoma in smokers. Drug Metab. Dispos. 2008, 36, 2316–2323.
- Sun, L.; Fan, X. Expression of cytochrome P450 2A13 in human non-small cell lung cancer and its clinical significance. J. Biomed. Res. 2013, 27, 202–207.
- Chiang, H.C.; Lee, H.; Chao, H.R.; Chiou, Y.H.; Tsou, T.C. Pulmonary CYP2A13 levels are associated with early occurrence of lung cancer-Its implication in mutagenesis of non-small cell lung carcinoma. Cancer Epidemiol. 2013, 37, 653–659.
- Fukami, T.; Nakajima, M.; Matsumoto, I.; Zen, Y.; Oda, M.; Yokoi, T. Immunohistochemical analysis of CYP2A13 in various types of human lung cancers. Cancer Sci. 2010, 101, 1024–1028.
- Timofeeva, M.N.; Kropp, S.; Sauter, W.; Beckmann, L.; Rosenberger, A.; Illig, T.; Jager, B.; Mittelstrass, K.; Dienemann, H.; Consortium, L.; et al. CYP450 polymorphisms as risk factors for early-onset lung cancer: Gender-specific differences. Carcinogenesis 2009, 30, 1161–1169.
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108.
- Li, Z.; Tuteja, G.; Schug, J.; Kaestner, K.H. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell 2012, 148, 72–83.
- Yu, X.; Gupta, A.; Wang, Y.; Suzuki, K.; Mirosevich, J.; Orgebin-Crist, M.C.; Matusik, R.J. Foxa1 and Foxa2 interact with the androgen receptor to regulate prostate and epididymal genes differentially. Ann. N. Y. Acad. Sci. 2005, 1061, 77–93.
- Shao, P.; Guo, N.; Wang, C.; Zhao, M.; Yi, L.; Liu, C.; Kang, L.; Cao, L.; Lv, P.; Xing, L.; et al. Aflatoxin G1 induced TNF-alpha-dependent lung inflammation to enhance DNA damage in alveolar epithelial cells. J. Cell. Physiol. 2019, 234, 9194–9206.
- Ling, G.; Wei, Y.; Ding, X. Transcriptional regulation of human CYP2A13 expression in the respiratory tract by CCAAT/enhancer binding protein and epigenetic modulation. Mol. Pharmacol. 2007, 71, 807–816.
- Wu, H.; Liu, Z.; Ling, G.; Lawrence, D.; Ding, X. Transcriptional suppression of CYP2A13 expression by lipopolysaccharide in cultured human lung cells and the lungs of a CYP2A13-humanized mouse model. Toxicol. Sci. 2013, 135, 476–485.




