
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katarina Borer | + 2906 word(s) | 2906 | 2021-11-03 07:52:49 | | | |
| 2 | Nora Tang | -10 word(s) | 2896 | 2021-11-08 02:34:42 | | | | |
| 3 | Dean Liu | Meta information modification | 2896 | 2021-11-11 04:58:43 | | | | |
| 4 | Nora Tang | + 217 word(s) | 3113 | 2021-11-11 05:04:52 | | |
Video Upload Options
Three powerful innate physiological defenses interfere with the maintenance of weight loss, whether it is achieved from the obese or healthy weight level. The first one, called adaptive thermogenesis, consists of persistent reduction in resting metabolic rate (RMR), the second one is increased hunger, and the third one is enhanced efficiency of energy storage. Adaptive thermogenesis was studied in individuals who lost substantial amounts of body weight and body fat in attempts to win “The biggest loser” televised competition.
1. Introduction
Since 1975, the worldwide rate of obesity has tripled [1], and in 2018, 42.4% and 31.1% of adult Americans were obese and overweight, respectively [2]. The unremitting increase over the past half century in the rate of overweight and obesity and their associated disabilities in U.S.A and other developed countries suggests that growth of obesity has epidemic features that require urgent mitigation. Without an attenuation of current weight gain trends, 1.35 billion people worldwide will be overweight and 573 million obese by 2030 [3]. Among the health problems usually listed as being associated with overweight and obesity are cardiovascular disease (CVD), hypertension, type 2 diabetes (T2D), hyperlipidemia, stroke, certain cancers, sleep apnea, liver and gall bladder disease, osteoarthritis, and gynecological problems [4][5]. There are also psychosocial consequences of obesity such as experience of weight stigma or perceived weight discrimination. These are associated with depression, anxiety, bulimia, body dissatisfaction, and low body and self-esteem [6][7][8]. Medical and surgical interventions against obesity have predominantly been applied to the most obese but have not been uniformly successful or without side effects. Behavioral interventions are hindered by psychosocial factors such as ingrained personal and family habits and societal customs.
The data presented in this review are based in part on PubMed and Google Scholar search for relevant supporting articles as well as the author’s research findings and views on the regulation of energy balance in humans [9][10][11][12][13][14][15][16][17][18][19]. There is insufficient understanding in four areas connected to obesity and its causes. The first one is inadequate understanding of the details of how obesity generates serious health problems. The second one is a general unawareness of the limitations of human physiology in control of weight gain and loss. The third one is how our misguided psychology toward eating contributes to overeating. Additionally, the fourth one is insufficient recognition of the features of developed societies that hinder efforts to control our weight.
An explanation of how obesity causes hormonal dysregulation of energy balance that results in insulin resistance, the key cause of obesity-linked pathologies, will be described first. In addressing the limitations of human physiology in control of weight gain and loss, evidence will be provided for the genetic basis of human appetite, predisposition for accumulation of fat, and absence of a negative-feedback mechanism of energy regulation. Regarding human psychological attitudes toward food, human seeking of palatable food, undisciplined eating, social facilitation of overeating, and opportunistic eating when overabundance of food is available at low cost, is examined. In addressing the societal factors that hinder human efforts to control body weight, data will be presented on the role of technological labor-saving developments, policies on dietary intake and housing patterns, and the efforts of profit motives of the food industry in promoting high-density palatable foods.
2. Limitations of Human Physiology in Controlling Weight Gain and Loss
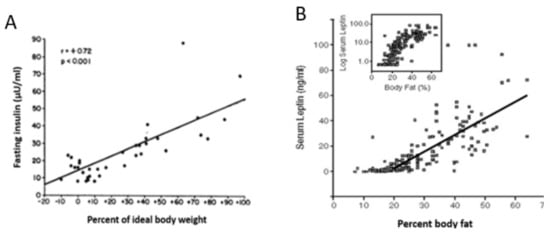
Point 1 : There is no negative-feedback regulation of body weight . An important fact that is not universally accepted is that the apparent stability of adult body weight in approximately half of non-obese American population is not a consequence of a regulated process based on negative-feedback compensations. This concept is unsettling as it alerts us that there is no automatic inborn mechanism for maintenance of healthy body weight. A number of hypotheses have unsuccessfully attempted to explain this apparent weight stability as representing a weight setpoint based on feedback adjustments in spontaneous food intake and physical activity [20][21]. As we are witnessing in the USA and other developed countries, there is no apparent negative feedback to prevent weight gain in overweight and obese individuals, although there is a robust increase in hunger and even energy-saving reduction in metabolic rate [22] with any significant weight gain. The popular formulation of the setpoint hypothesis is based on the expectation that the negative-feedback signal that encodes decreases in adipose tissue mass is a reduction in the circulating concentration of the hormone leptin released from the subcutaneous WAT [23]. This hypothesis was triggered in part by the observation that leptin injections reduce hunger and produce weight loss in obese humans genetically unable to produce leptin [24]. The expectation that the same relationship operates in neurologically normal humans did not materialize. In a large trial where obese individuals were injected with several doses of leptin ranging from sub-threshold to supra-physiological levels, there was no effect on appetite or weight loss [25]. In addition, leptin concentrations rise in both humans and animals parallel with the rise in obesity ( Figure 1 ), a clear demonstration that this hormone does not operate as a weight-normalizing negative-feedback signal. It is more likely that injected leptin’s effectiveness in causing weight loss in congenital leptin insufficiency is due to its lipolytic properties and its role in counter-regulating the obesifying insulin action [12].
Point 3. Hunger and satiation are mediated by oral and gastrointestinal signals and not by circulating metabolites or hormones . There have been repeated attempts to link hunger either to changes in circulating metabolites such as glucose, ketones, or FFAs, or to hormones such as leptin and insulin (the putative satiety hormones [23][24]) or to ghrelin as the putative hunger hormone [26]. Neither hypothesis has withstood testing. The assumption that circulating metabolites or nutrients influence hunger was contested in two studies [18][27]. In the first one [18], hunger was measured in response to different size meals (100 vs. 500 kcall) taken by mouth but with intravenous supplementation of small meals with parenteral nutrients. Hunger was also tested when the large meal was combined with exercise which depleted close to 90 percent of ingested calories. Again, the energy shortfall was compensated by intravenous infusion of nutrients. As Figure 2 clearly shows, only the size of meals ingested by mouth and processed by the gastrointestinal tract influenced hunger and satiation. With the 100 kcal meal, the fullness rating was lower ( Figure 2 , bottom left panel), and hunger much greater (top left panel), than with the 500 kcal meal. Intravenous infusion of nutrients of similar composition to that of orally eaten meals, did not influence either the hunger or satiation. In a similar vein, calories expended by exercise were not detected and reflected in increased hunger (top right panel) or in reduced satiation (bottom right panel). Only a variation in the size of the meal eaten by mouth and processed through the gastrointestinal tract affected the magnitude of hunger and satiation and remained unaffected by supplementation of intravenous calories or by exercise energy expenditure.
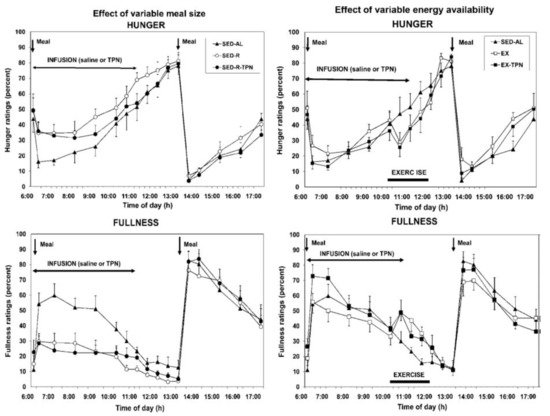
The second study [27] confirmed that increases in circulating metabolic fuels affected by exercise in fasted state, or by calories absorbed from meals during post-meal exercise, had no impact on hunger and satiation ratings. Finally, the confirmation that satiation reflects the volume of ingested food and not their caloric content was demonstrated in a study where healthy volunteers were provided for 11 weeks with identical diets that differed only in fat content, a higher-fat diet containing 30 to 35% fat, or lower-fat diet with 20 to 25% fat [28]. Both groups consumed approximately the same daily volume of food (between 1400 and 1450 g) but did not adjust the quantity eaten to account for the difference in dietary fat content. As a result, the body weights of the two groups diverged. Finally, while hunger and satiation responded only to quantity of orally ingested food, the putative satiety hormones insulin and leptin responded to the state of all circulating fuels, the absorbed food that was eaten, infused parenteral nutrition, and calories lost through exercise [18] ( Figure 3 ). However, the concentrations of these hormones, which tracked fuel availability in circulation, did not affect hunger or satiation.
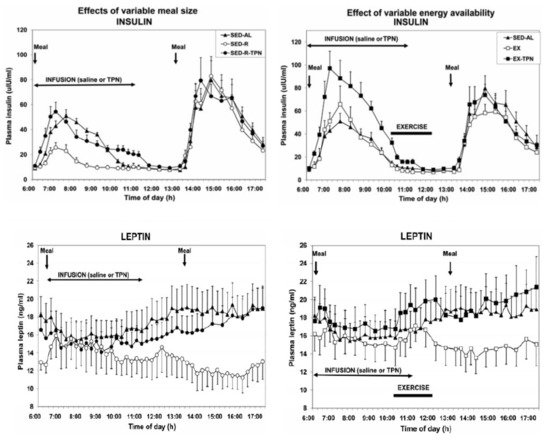
Point 5. Adaptive thermogenesis and increases in insulin sensitivity and hunger hamper loss of body fat . An inconvenient finding for most individuals trying to lose weight is that deliberate weight loss through food restriction, with or without added exercise, is regained in the matter of months or years [29]. Three powerful innate physiological defenses interfere with the maintenance of weight loss, whether it is achieved from the obese or healthy weight level. The first one, called adaptive thermogenesis, consists of persistent reduction in resting metabolic rate (RMR) [30], the second one is increased hunger, and the third one is enhanced efficiency of energy storage. Adaptive thermogenesis was studied in individuals who lost substantial amounts of body weight and body fat in attempts to win “The biggest loser” televised competition. In comparison with the weight loss produced by Roux-en-Y gastrectomy, with both producing a loss of between 40 and 49 kg, respectively, and with a smaller 16% loss of lean body mass in the televised competition [31], RMR decreased more in both groups than expected based on measured body composition changes. The magnitude of this metabolic adaptation was correlated with the magnitude of energy imbalance and the decrease in circulating leptin. The persistence of this adaptive thermogenesis response was revealed after 6 years when 16 of the “Biggest Looser” competitors were re-tested [22]. They regained 41 kg of 58 kg lost, but their RMR remained 500 kcal/day below the expected level, representing an innate physiological defense against weight loss. This response is currently interpreted as an evolutionary defense against a reduction in total daily energy expenditure [31]. In this view, higher energy expenditure due to increased body mass is sustained with higher energy intake, but any reduction in intake, increase in exercise energy expenditure, and reduction in body mass is compensated by a reduction in RMR. The other two innate processes that interfere with weight-loss maintenance and promote weight regain are a large increase in the efficiency of energy utilization and increased hunger. Through reduction in the size of adipocytes and increase in the density of insulin receptors on their cell membranes, weight loss significantly increases insulin sensitivity and thus the effectiveness of its four actions. Insulin now more powerfully promotes nutrient uptake, glycogen and fat synthesis, and blocks fuel store degradation. Fasting plasma leptin is now reduced to very low levels ( Figure 1 ) which reduce satiation and promote hunger. This role of leptin as a “starvation” hormone has been demonstrated by administering the hormone to subjects who have experimentally undergone a 10% weight loss. Leptin administration suppressed their hunger and helped maintain their weight loss [32].
3. Limitations of Human Psychology in Controlling Weight Gain and Loss
Point 1: We can consciously control our seeking of palatable odors and tastes . Although we have an inborn preference for the sweet taste, and dislike for bitter and sour taste manifested at birth [33], we discriminate between seeking food when we are hungry and desiring food that smells and tastes good. Hunger produces a deliberate “wanting” of a food reward, while our motivation for palatable odors and tastes represents our “liking” or desiring food reward [34]. Substantial orbitofrontal and insular cortical and limbic circuits have been identified as a substrate of the hunger motivation in response to negative energy balance and inadequate consumption of food. Interspersed within this circuitry, and centered in the mesolimbic nucleus accumbens, are neural substrates of hedonic motivation that can amplify human seeking of palatable tastes and odors which operate with dopamine and opioids as neurotransmitters. Beyond the motivation for palatability, the appeal of food is enhanced also by its variety, which can increase intake by as much as 29% [35]. We acknowledge our liking of palatable food and of its variety through the practice of gastronomy. Humans practice gastronomy and accommodate the desire for palatability daily in the way they sequence palatable foods in their meals to progress from salty and savory to maximally palatable sweets at the end.
Point 3. Humans eat more in company of others . Social facilitation of food intake, a term coined by John De Castro [36], is a phenomenon humans share with a number of other animals such as dogs [37] and chickens [38]. Social facilitation implies that the amount eaten by humans in spontaneously ingested meals is positively correlated with the number of other people present. Socially facilitated food intake can increase by 44% and is related to the duration of meals and not to an increase in hunger. Others in the group can be strangers such as when eating in a restaurant [39], or they may represent virtual company as in food commercials seen on television [40]. Another variable in social settings that increases the amount eaten is matching the speed of eating seen in other people [41].
Point 4. Opportunistic eating as a function of food quantity . Humans eat more food when it is provided in larger quantities. This takes several forms: (1) more is eaten with larger food portions whether presented during meals [42] or available in packages [43]; (2) more is eaten from larger containers [44]; and more is eaten in response to advantageous price incentives offered by fast-food companies. Higher-calorie combination meals in fast-food restaurants offer significantly more calories per dollar compared to regular meals, suggesting there is a strong financial incentive for consumers to ‘upsize’ their orders [45].
Psychological factors contributing to overeating can be managed by first understanding their causes. Predilection for palatable food can be rationally controlled by enjoying good tasting food without allowing its available quantity to dictate how much we eat. We need to expect and prepare for oversized servings in restaurants, food packaging that misrepresents the contained calories, and social situations or celebrations that promote food overconsumption. Simply understanding that we tend to eat unnecessary meals and snacks over an extended wakeful period has another clear solution by establishing a time-restricted feeding pattern [46]. Limiting food intake to a 6 to 10 h daily time period reduced food intake, weight gain, and caused fat loss without eliciting excessive hunger both in obese and diabetic mice [47] ovariectomized mice [48], in obese [49] and pre-diabetic humans [50], and in obese postmenopausal women [51], and therefore can be implemented to prevent overeating and excessive weight gain.
4. Features of Developed Societies That Hinder Efforts to Control Our Weight
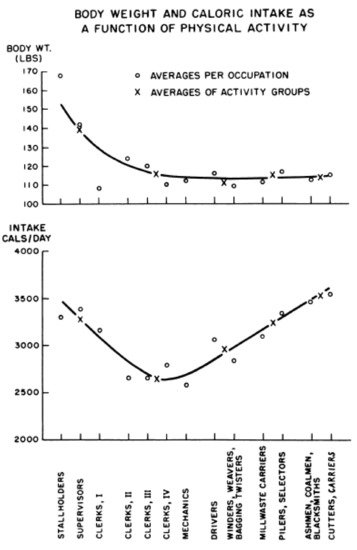
Large human brains have contributed to development of technologies, structured the built environments, instituted societal policies, and fostered economic growth, the first two of which have reduced the need for physical work and the other three have facilitated food overconsumption. Labor-saving devices have brought humanity, especially in developed countries, to the non-homeostatic range of interactions between weight gain and physical activity (left part of Figure 4 ).
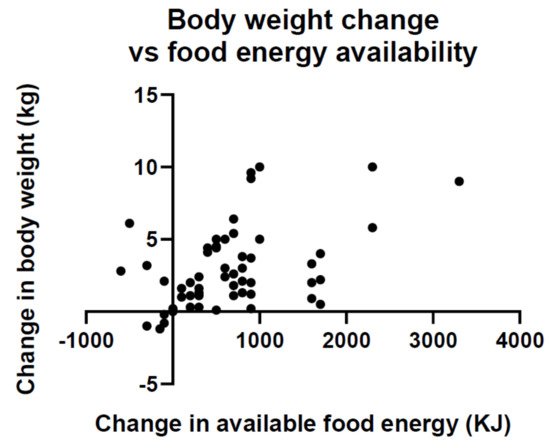
Point 4. Economic growth and development brings about an overabundance of inexpensive foods that foster overconsumption and weight gain . A byproduct of booming economic growth is an overabundance of moderately priced food. A global analysis has found that in 69 countries an increase in food energy supply over a 4 year interval was associated with a significant increase in average body weight ( Figure 5 ) [52][53]. The magnitude of association was sufficient to explain population weight gain in the past 50 years.
Point 5. The food industry is guided more by profit motive than by considerations of human health in aggressively promoting high-density palatable foods . The food in US and developed countries was traditionally produced locally for neighborhood markets and with relatively little processing. Current system involves global suppliers to maximize efficiency, reduce costs, and increase production and profit before the food reaches the consumer. Supermarkets and the growing fast-food industry have introduced a variety of energy-dense processed foods, sometimes called ultra-processed foods because of their high content of palatable sugar and saturated fats. This is becoming a major source of energy in developed and developing countries and is seen as a driver of the obesity epidemic [54][55]. Spiking of processed food with additional sugar, fat, and salt increases its palatability, quantity eaten, and marketability, and is associated with development of psychological dependence or even addiction to such food. Eating such food is also associated with a number of health pathologies [54]. Ultra-processed food increases the risk of overeating not only because of its palatability, but also because of its high caloric density reflecting human dependence on ingested food volume for satiation, and not its caloric density. Of 22,659 adults whose caloric intakes of processed food and weight changes were monitored over a 5 year period by UK Biobank, 947 were obese and 1900 had abdominal obesity [55]. Participants in the highest quartile of ultra-processed food consumption had a 79% higher risk of developing overall obesity, 31% higher risk of experiencing a ≥5% increase in BMI, 35% increased risk of increased waist circumference, and 30% increased risk for abdominal obesity. They also had a 14% increased risk of greater percent body fat than individuals in the lowest quartile of consumption. It is estimated that factors leading to poor diet produce a bigger health burden than tobacco, alcohol, and inactivity put together [56]. Yet, influencing the food industry to change its profit-centered advertising and selling of ultra-processed food remains difficult [5]. The argument promoted by the food industry against regulation over its business strategies has focused on insisting that individuals are responsible for conscious control over their food choices [57][58].
Relative to computer and smartphone-based approaches to weight control, a very simple device, a bathroom scale, has an outsize effect in curbing weight gain [59]. Its efficacy has been documented in late 1980s and subsequently in 1990s [60] when it was reported that a majority of people registered in National Weight Control Registry, who lost 30 lb and maintained this weight loss for an average of 5 years, weighed themselves several times a week. Combining daily weighing against a chart documenting weight-loss progress led not only to a weight loss, but also prevented weight regain [61].
References
- World Health Organization. The WHO STEPwise Approach to Noncommunicable Disease Risk Factor Surveillance (STEPS); WHO: Geneva, Switzerland, 2011.
- Fryar, C.; Carroll, M.D.; Afful, J. Prevalence of Overweight, Obesity, and Severe Obesity among Adults Aged 20 and Over: United States, 1960–1962 through 2017–2018; NCHS Health E-Stats; U.S. National Center for Health Statistics: Hyattsville, MD, USA, 2020.
- Kelly, T.; Yang, W.; Chen, C.-S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437.
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88.
- Nestle, M.; Jacobson, M.F. Halting the obesity epidemic: A public health policy approach. Public Health Rep. 2000, 115, 12–24.
- Hatzenbuehler, M.L.; Keyes, K.M.; Hasin, D.S. Associations Between Perceived Weight Discrimination and the Prevalence of Psychiatric Disorders in the General Population. Obesity 2009, 17, 2033–2039.
- Puhl, R.; Suh, Y. Stigma and Eating and Weight Disorders. Curr. Psychiatry Rep. 2015, 17, 1–10.
- Vartanian, L.R.; Novak, S.A. Internalized Societal Attitudes Moderate the Impact of Weight Stigma on Avoidance of Exercise. Obesity 2011, 19, 757–762.
- Borer, K.T. Physical activity in the prevention and amelioration of osteoporosis n women: Interaction of mechanical, hormonal, and dietary factors. Sports Med. 2005, 35, 779–830.
- Borer, K.T. How effective is exercise in producing fat loss? Kinesiology 2008, 40, 127–138.
- Borer, K.T. Nonhomeostatic Control of Human Appetite and Physical Activity in Regulation of Energy Balance. Exerc. Sport Sci. Rev. 2010, 38, 114–121.
- Borer, K.T. Counterregulation of insulin by leptin as key component of autonomic regulation of body weight. World J. Diabetes 2014, 5, 606–629.
- Borer, K.T. Understanding Human Physiological Limitations and Societal Pressures in Favor of Overeating Helps to Avoid Obesity. Nutrients 2019, 11, 227.
- Borer, K.T. How does exercise support dietary approaches to weight loss and better health? Ann. Kinesiol. 2019, 10, 31–58.
- Borer, K.T.; Zheng, Q.; Jafari, A.; Javadi, S.; Kernozek, T. Nutrient Intake Prior to Exercise Is Necessary for Increased Osteogenic Marker Response in Diabetic Postmenopausal Women. Nutrients 2019, 11, 1494.
- Borer, K.T.; Potter, C.D.; Fileccia, N. Basis for the hypoactivity that accompanies rapid weight gain in hamsters. Physiol. Behav. 1983, 30, 389–397.
- Borer, K.T.; Wuorinen, E.; Chao, C.; Burant, C. Exercise energy expenditure is not consciously detected due to oro-gastric, not metabolic, basis of hunger sensation. Appetite 2005, 45, 177–181.
- Borer, K.T.; Wuorinen, E.; Ku, K.; Burant, C. Appetite Responds to Changes in Meal Content, Whereas Ghrelin, Leptin, and Insulin Track Changes in Energy Availability. J. Clin. Endocrinol. Metab. 2009, 94, 2290–2298.
- Lin, P.-J.; Borer, K.T. Third Exposure to a Reduced Carbohydrate Meal Lowers Evening Postprandial Insulin and GIP Responses and HOMA-IR Estimate of Insulin Resistance. PLoS ONE 2016, 11, e0165378.
- Farias, M.M.; Cuevas, A.M.; Rodriguez, F. Set-Point Theory and Obesity. Metab. Syndr. Relat. Disord. 2011, 9, 85–89.
- Müller, M.J.; Geisler, C.; Heymsfield, S.B.; Bosy-Westphal, A. Recent advances in understanding body weight homeostasis in humans. F1000Research 2018, 7, 1025.
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 2016, 24, 1612–1619.
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671.
- Farooqi, S.; Keogh, J.M.; Kamath, S.; Jones, S.; Gibson, W.; Trussell, R.; Jebb, S.A.; Lip, G.Y.H.; O’Rahilly, S. Partial leptin deficiency and human adiposity. Nature 2001, 414, 34–35.
- Heymsfield, S.B.; Greenberg, A.S.; Fujioka, K.; Dixon, R.M.; Kushner, R.; Hunt, T.; Lubina, J.A.; Patane, J.; Self, B.; Hunt, P.; et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA 1999, 282, 1568–1575.
- Cummings, D.E. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol. Behav. 2006, 89, 71–84.
- Borer, K.T.; Lin, P.-J.; Wuorinen, E. Timing of meals and exercise affects hormonal control of glucoregulation, insulin resistance, substrate metabolism, and gastrointestinal hormones but has little effect on appetite in postmenopausal women. Nutrients 2021. (manuscript under review).
- Kendall, A.; Levitsky, D.A.; Strupp, B.J.; Lissner, L. Weight loss on a low-fat diet: Consequence of the imprecision of the control of food intake in humans. Am. J. Clin. Nutr. 1991, 53, 1124–1129.
- Wing, R.R.; Espeland, M.A.; Clark, J.M.; Hazuda, H.P.; Knowler, W.C.; Pownall, H.; Unick, J.; Wadden, T.; Wagenknecht, L.; for the Action for Health in Diabetes (Look AHEAD) Study Group. Association of Weight Loss Maintenance and Weight Regain on 4-Year Changes in CVD Risk Factors: The Action for Health in Diabetes (Look AHEAD) Clinical Trial. Diabetes Care 2016, 39, 1345–1355.
- Knuth, N.D.; Johannsen, D.L.; Tamboli, R.A.; Marks-Shulman, P.A.; Huizenga, R.; Chen, K.; Abumrad, N.N.; Ravussin, E.; Hall, K.D. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity 2014, 22, 2563–2569.
- Pontzer, H. Constrained total energy expenditure and the evolutionary bioogy of energy expenditure. Exerc. Sports Sci. Rev. 2015, 43, 110–116.
- Rosenbaum, M.; Leibel, R.L. 20 years of leptin: Role of leptin in energy homeostasis in humans. J. Endocrinol. 2014, 223, T83–T96.
- Crook, C. Taste perception in the newborn infant. Infant Behav. Dev. 1978, 1, 52–69.
- Berridge, K.C. ‘Liking’ and ‘wanting’ food rewards: Brain substrates and roles in eating disorders. Physiol. Behav. 2009, 97, 537–550.
- Raynor, H.A.; Vadiveloo, M. Understanding the Relationship Between Food Variety, Food Intake, and Energy Balance. Curr. Obes. Rep. 2018, 7, 68–75.
- De Castro, J.M. Social facilitation of duration and size but not rate of the spontaneous meal intake of humans. Physiol. Behav. 1990, 47, 1129–1135.
- Ross, S.; Ross, J.G. Social Facilitation of Feeding Behavior in Dogs: I. Group and Solitary Feeding. Pedagog. Semin. J. Genet. Psychol. 1949, 74, 97–108.
- Strobel, M.G.; Macdonald, G.E. Induction of eating in newly hatched chicks. J. Comp. Physiol. Psychol. 1974, 86, 493–502.
- Nguyen, B.P.; Powell, L.M. The impact of restaurant consumption among US adults: Effects on energy and nutrient intakes. Public Health Nutr. 2014, 17, 2445–2452.
- Harris, J.L.; Bargh, J.A.; Brownell, K.D. Priming effects of television food advertising on eating behavior. Health Psychol. 2009, 28, 404–413.
- Dongen, M.V.-V.; Kok, F.J.; de Graaf, C. Eating rate of commonly consumed foods promotes food and energy intake. Appetite 2011, 56, 25–31.
- Rolls, B.J.; Morris, E.L.; Roe, L.S. Portion size of food affects energy intake in normal-wight and overweight men and women. Am. J. Clin. Nutr. 2002, 76, 1207–1213.
- Rolls, B.J.; Roe, L.S.; Kral, T.V.; Meengs, J.S.; Wall, D.E. Increasing the portion size of a packaged snack increases energy intake in men and women. Appetite 2004, 42, 63–69.
- Wansink, B.; Kim, J. Bad Popcorn in Big Buckets: Portion Size Can Influence Intake as Much as Taste. J. Nutr. Educ. Behav. 2005, 37, 242–245.
- Vercammen, K.A.; Frelier, J.M.; Moran, A.J.; Dunn, C.G.; Musicus, A.A.; Wolfson, J.; Ullah, O.S.; Bleich, S.N. Understanding price incentives to upsize combination meals at large US fast-food restaurants. Public Health Nutr. 2020, 23, 348–355.
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059.
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic inter-vention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005.
- Omotola, O.; Legan, S.; Slade, E.; Adekunle, A.; Pendergast, J.S. Estradiol regulates daily rhythms underlying diet-induced obesity in female mice. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1172–E1181.
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syn-drome. Cell Metab. 2020, 31, 92–104.
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732.
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Lin, S.; Varady, K.A. Changes in body weight and metabolic risk during time restricted feeding in premenopausal versus postmenopausal women. Exp. Gerontol. 2021, 154, 111545.
- Vandevijvere, S.; Chow, C.C.; Hall, K.D.; Umali, E.; Swinburn, B.A. Increased food energy supply as a major driver of the obesity epidemic: A global analysis. Bull. World Health Organ. 2015, 93, 446–456.
- Zobel, E.H.; Hansen, T.; Rossing, P.; von Scholten, B.J. Global Changes in Food Supply and the Obesity Epidemic. Curr. Obes. Rep. 2016, 5, 449–455.
- Lustig, R. Ultraprocessed Food: Addictive, Toxic, and Ready for Regulation. Nutrients 2020, 12, 3401.
- Rauber, F.; Chang, K.; Vamos, E.P.; da Costa Louzada, M.L.; Monteiro, C.A.; Millett, C.; Bertazzi Levy, R. Ultra-processed food consumption and risk of obesity: A prospective cohort study of UK Biobank. Eur. J. Nutr. 2021, 60, 2169–2180.
- WHO. Global Action Plan for the Prevention and Control of NCDs 2013–2020; World Health Organization: Geneva, Switzerland, 2013; Available online: http://www.who.int/nmh/events/ncd_action_plan/en/ (accessed on 12 September 2021).
- Stuckler, D.; McKee, M.; Ebrahim, S.; Basu, S. Manufacturing epidemics: The role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med. 2012, 9, e1001235.
- Capewell, S.; Lloyd-Williams, F. The role of food industry in health: Lessons from tobbacco? Br. Med. Bull. 2018, 125, 131–143.
- Pacanowski, C.R.; Bertz, F.; Levitsky, D.A. Daily self-weighing to control body weight in adults: A critical review of the literature. SAGE Open 2014, 4, 1–16.
- McGuire, M.T.; Wing, R.R.; Klem, M.L.; Seagle, H.M.; Hill, J.O. Long-term maintenance of weight loss: Do people who lose weight through various weight-loss methods use different behaviors to maintain their wight? Int. J. Obes. 1998, 22, 572–577.
- Pacanowski, C.R.; Levitsky, D.A. Frequent Self-Weighing and Visual Feedback for Weight Loss in Overweight Adults. J. Obes. 2015, 2015, 1–9.




