Recent research and development efforts around diploid progenitor lung cells (e.g., FE002-Lu or Walvax-2 sources) consist in qualification for potential use as optimal and renewed vaccine production substrates and, alternatively, for potential therapeutic applications in respiratory tract regenerative medicine. Potentially effective, safe, and sustainable cell therapy approaches for the management of inflammatory lung diseases or affections and related symptoms (e.g., COVID-19 patients and burn patient severe inhalation syndrome) using local homologous allogeneic cell-based or cell-derived product administrations are considered.
1. Introduction
Vast historical experience has been gathered around industrial use of diploid progenitor cell sources (e.g., MRC-5, WI-38 cell types) as vaccine substrates since the 1960s, when developmental cell biology studies served as a basis for optimization of biological starting material selection [1][2][3][4][5][6][7]. Despite extensive use of such materials, as well as ethical and moral debates centered mainly upon the original tissue procurement, such diploid cells have been instrumental in the development and therapeutic application of several life-saving products over a half-century [8][9][10][11][12][13][14][15][16][17][18]. The increasing material demand driven by modern biotechnological industrial development and aging of the original cell sources (i.e., stability issues) has recently prompted renewed consideration for novel substrate cell type establishment and qualification [19][20][21][22]. The unique sustainability and safety aspects of diploid progenitor cell sources, exploited as safe and stable multi-tiered biobanks, are emerging as critical advantages in modern manufacturing and quality assurance environments [23][24][25][26]. Most importantly, and in addition to the considerable inherent technical advantages of such cellular materials, a vast therapeutic potential exists for the use of diploid progenitor cells or cell derivatives for application in allogeneic regenerative medicine [27][28][29][30][31][32]. Indeed, skin-derived diploid progenitor cell sources have been extensively studied and clinically applied, in particular for the therapeutic management of pediatric burns and chronic inflammatory cutaneous wounds [33][34][35][36][37][38][39][40][41][42][43][44].
Optimized methodological aspects concerning tissue procurement and processing for progenitor cell isolation were the foundations of recent work on cutaneous and musculoskeletal cell therapies [25][31][45][46][47][48][49][50]. Based on available technical experience in diploid progenitor cell GMP manufacture upscaling and transposition, current efforts are directed toward the qualification and appropriate homologation of recently isolated cells as biotechnological substrates for optimal renewal and eventual replacement of original cell stocks [32][42]. Furthermore, therapeutic approaches for the management of inflammatory lung diseases or affections and related symptoms (e.g., COVID-19 patients and burn patient severe inhalation syndrome) using local homologous allogeneic cell-based or cell-derived product administrations are considered.
2. Renewal of Vaccine Substrates Using Original Seed Stocks or Modern Diploid Progenitor Cell Types
Despite the high sustainability and stability of the original diploid cell banks established in the 1960s, specific technical problems have arisen as even the best cryopreservation storage practices have not made the preserved vial impervious to the effects of time. With the example of the MRC-5 cell source, the importance of the historic use of such cells has been brought to the attention of the World Health Organization (WHO) for sustainable exploitation of the remaining materials. Therefore, the original PDL 7 stock (i.e., population doubling level 7) established in 1966 was recently renewed over concerns of deteriorating quality of original glass ampoules and related stability or biosafety risks
[22]. The MRC-5 PDL 13 seed bank was therefore established by the National Institute for Biological Standards and Control (NIBSC) and considered for homologation as a WHO reference cell bank in order to be able to sustainably (i.e., during several decades) provide substrates for vaccine product development and manufacture (i.e., in particular for validation and testing phases)
[51]. Materials may be provided directly to individual manufacturers, which bare the responsibility of developing and qualifying specific master cell banks (MCB) and working cell banks (WCB) for their own use.
In parallel to the renewal of seed stocks of existing cell types, current efforts are also allocated toward the establishment of novel cell types within modern legal workflows and technical capabilities
[45][46][47][48][50]. The objective would be to develop more extensively traced cell sources, with the development of a seed stock or parental cell bank (PCB) at very early passage levels, in order to increase overall security by using modern cell culture and testing technologies, as well as validated methodological workflows (
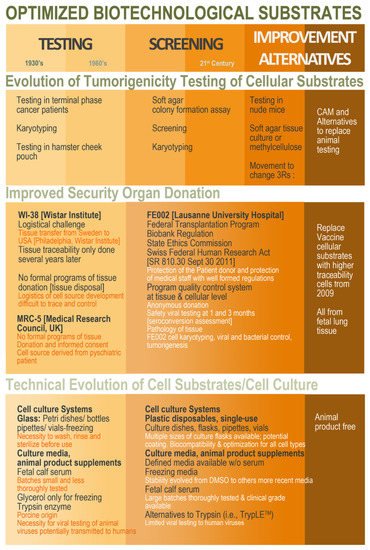
Figure 1).
Figure 1. Schematic overview of the ethical, legal, and technical parameter evolution around the establishment of cell sources to be characterized and qualified for use as vaccine production substrates. Overall, hindsight and experience gathered from historic industrial practice may be conjugated with modern regulatory and technical workflows in order to develop optimized (i.e., safe, sustainable, and efficient) biotechnological substrates obtained in modern regulatory settings and methodological workflows. CAM, chorioallantoic membrane; DMSO, dimethyl sulfoxide.
Despite numerous advantages of such modern approaches (e.g., full safety assessment, documentation of donor consent, use of universal cell stocks), high inertia exists within manufacturer and regulatory circles with regard to the homologation of novel cell types. A noteworthy example of modern diploid cell type establishment is the Chinese Walvax-2, derived from prenatal lung tissue and proposed in 2015 (Wuhan, China) as a qualified substrate for viral material propagation
[20]. The need for high quantities of cell substrates to satisfy the Chinese industrial demand, along with restrictions on the availability of imported MRC-5 cells, prompted the development of this new diploid cell source. Specifically, the Chinese Pharmacopoeia (Volume III, 2010) limits the use (i.e., for vaccine manufacturing) of human diploid cell strains to two-thirds of the qualified
in vitro lifespan, rendering the dependency on imported cell sources intolerable in terms of supply chain risk management
[20].
For the isolation and establishment of the Walvax-2 cell source, the original methods of Hayflick (i.e., 1:2 dilution for cell culture procedures) were replicated
[1][20]. Although this procedure enables the development of large stocks of cells, it is possible to further maximize production with the implementation of optimized technical specifications, which would be of high interest for extensive up-scaling needs
[25].
Overall, vast opportunities for vaccine substrate renewal exist and have been underlined by current shortages, themselves exacerbated by the COVID-19 pandemic. Novel diploid cell sources have been developed, similar to historically used materials, with prenatal and perinatal tissues (e.g., Walvax-2 diploid human lung cells, CCRC-1 hUC-MSCs, and diploid human amniocytes). In particular, based on the large available data on human diploid progenitor cell GMP banking in Switzerland for therapeutic material sourcing, high interest is currently set locally on specific cell types (i.e., FE002-Lu lung diploid progenitor cell types) for candidacy as novel and robust cell source establishment
[25]. Indeed, specific requirements for biological material sourcing, cell type establishment, and cell banking have already been met for FE002 diploid cell types for use thereof as active pharmaceutical ingredients, showing strong overlaps with the requirements set forth for vaccine substrates
[31][32][33][42][43][44][45][52][53][54]. To illustrate these parallels, a comparative analysis of MRC-5 cells and FE002-Lu cells was performed and summarized (
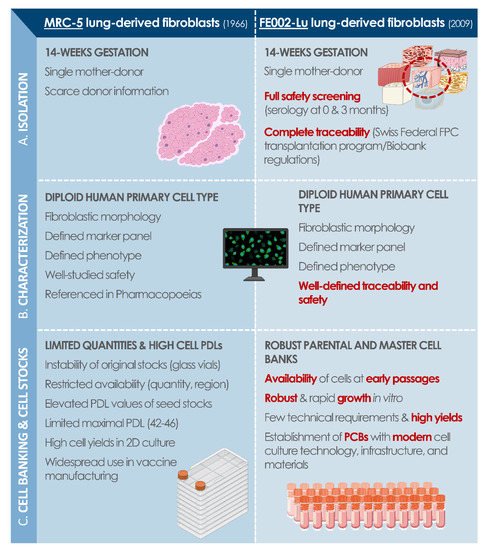
Figure 2). In particular, modern methodological and technical approaches to original cell sourcing appear as critical and key points for justification of regulatory compliance of considered modern human diploid cell sources.
Figure 2. Modern cell source replacement potential, with a comparative assessment of MRC-5 and FE002-Lu diploid cell types, which were both established following cell isolation from prenatal lung tissue (i.e., 14 weeks of gestation). (A) Isolation of primary cells from donated tissue samples for initiation of in vitro culture. (B) Characterization data related to established cell type quality attributes. (C) Technical overview of the similarities and differences between both considered cell sources in terms of cell culture and cell banking. FPC, fibroblast progenitor cells; PCB, parental cell bank; PDL, population doubling level.
3. Multi-Tiered Cell Banking of Diploid Lung Tissue-Derived Progenitors
Various technical methodologies for lung tissue-derived progenitor cell isolation and culture initiation may be adopted (e.g., mechanical dissociation or trypsin-based tissue digestion), whereas the official context or framework for tissue sourcing and procurement should be adequately defined (e.g., transplantation programs), as described previously
[25].
Following strict procedures and controlled access to materials and information, prenatal lung tissue samples are anonymously yet traceably obtained for subsequent bioprocessing, ensuring optimal quality and safety of progeny cell sources. Technical specificities of lung progenitors place such cell sources at the forefront of potential therapeutic material candidates for regenerative medicine applications. Indeed, rapid establishment and extensive cell banking capabilities practically negate the need for repeated organ donations, as attested by the industrial use of the MRC-5 cell type, for example, which has lasted over fifty years thus far following a single tissue donation
[16]. Therefore, repeat testing and validation of new cell sources or pooling thereof are not required, and this aspect contributes to augment safety and to lower overall manufacturing costs. With regard to technical aspects of primary lung progenitor cell isolation, parental cell banks (PCB) at early passages may be rapidly established, composed of several dozen vials, each containing 10
6 to 10
7 cells to be preserved
[32]. Standard culture media (e.g., DMEM supplemented with fetal bovine serum) and culture conditions (e.g., humidified atmosphere under 5% CO
2 and 37 °C incubation) are sufficient for initiation of adherent lung tissue-derived progenitor cell cultures
in vitro.
Due to the high robustness and extensive proliferative potential of primary progenitor cells, such sources may be used in industrial-scale cell banking campaigns under good manufacturing practice (GMP) requirements
[41]. For an optimal and sustainable use of progenitor cell biobanks, serial expansions may be performed for the eventual establishment of multi-tiered cryopreserved stocks of cells (
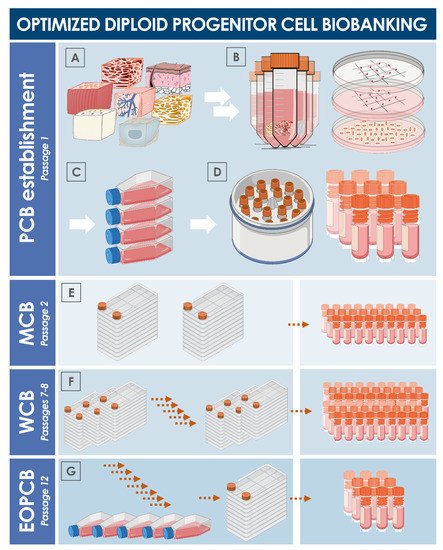
Figure 3). Inherent characteristics of diploid progenitor cell types enable thorough iterative testing and validation steps to be performed on all cell bank tiers, which may be allocated into parental cell banks (PCB), master cell banks (MCB), and working cell banks (WCB). Additionally, end of production cell banks (EOPCB) may be generated for technical and safety qualification of the cell types of interest. Culture reagents and conditions described hereabove for primary cell isolation remain the same for subsequent expansions, yet extensive optimization must be carried out in a pilot cell banking campaign to define, among other parameters, the optimal culture vessels (i.e., type, model, surface, oxygenation method), media supplement source (i.e., fetal bovine serum supplier and lot number), and culture maintenance workflows (i.e., culture medium volumes, medium exchange rates, cell seeding densities, cell harvesting confluency, culture period duration). Once optimal technical specifications have been established, large-scale cell banking may be tangibly performed. Stringent in-process controls and material testing must be adapted for quality assurance purposes. At each step of the cell banking process, adequate product characterization and release testing must be performed on manufacturing lots (e.g., recovery assays, isoenzyme testing and DNA fingerprinting, sterility testing, or research of microorganisms with a particular focus set of viruses of human, bovine, and porcine origin). Additional testing performed on EOPCB materials (e.g.,
in vitro and
in vivo tumorigenicity assays, karyology studies) enable the qualification of the safety and stability of considered progenitor cell types
[55][56][57][58][59].
Figure 3. Schematic overview of the technical steps to be undertaken for primary diploid cell type establishment and serial culture expansion for multi-tiered cell bank establishment. (A) Procurement of specific starting biological materials. (B) Enzymatic or mechanical cell dissociation. (C) Preliminary expansion in cell culture vessels. (D) Cryopreservation and constitution of a parental cell bank. (E) Manufacture of master cell banks. (F) Manufacture of working cell banks. (G) Manufacture of an end of production cell bank. Exhaustive documentation of the successive steps and thorough characterization, qualification, and release testing schemes allow for the safe and sustainable use of considered diploid cell sources. EOPCB, end of production cell bank; MCB, master cell bank; PCB, parental cell bank; WCB, working cell bank.