The herpesvirus partial DNA polymerase gene was detected using polymerase chain reaction in oropharyngeal swabs of 16 out of 170 owls examined that were captured in or near nest boxes. Herpesvirus was detected in Ural owls (Strix uralensis), in both adults and young, but not in tawny owls (Strix aluco). In yellow-necked mice (Apodemus flavicollis), as the main prey of tawny owls and Ural owls in the area, herpesvirus was detected in the organs of 2 out of 40 mice captured at the same locations as the owls. Phylogenetic analysis showed that the herpesvirus sequences detected in the Ural owls differed from the herpesvirus sequences detected in the yellow-necked mice. The results indicate that herpesvirus infection exists in the breeding wild Ural owl population. However, herpesvirus-infected owls did not show any clinical or productivity deviances and, based on a phylogenetic comparison of detected herpesvirus sequences and sequences obtained from Genbank database, it seems that mice and other rodents are not the source of owl infections. The most probable transmission pathway is intraspecific, especially from adults to their chicks, but the origin of herpesvirus in owls remains to be investigated.
1. Introduction
Some pathogens pose significant natural hazards for wild bird populations
[1], and they even have potentials for outbreaks in humans, especially when ecosystems and thus regulatory ecosystem services are depleted
[2]. Raptors, as predators at the top of the food chain, are particularly good environmental sentinels for detection of wildlife zoonosis
[3]. However, the prevalence, transmission, and impacts of viruses in free-ranging raptors are still a poorly understood phenomenon, and this is probably reflected in cumulative effects in raptor mortality and fecundity combined with other environmental impacts
[4][5], as well as individual variation
[6].
Diverse herpesviruses have frequently been found in different free-living bird species
[7][8][9][10][11]. In owls, herpesvirus was discovered in the 1970s
[12], later known as Strigid herpesvirus (StHV 1), and it has been reported in captive and free-ranging owls in Asia, Europe, North America, and Australia
[10]. General, all avian herpesviruses are members of the genera
Iltovirus and
Mardivirus of the subfamily Alphaherpesvirinae. However, many viruses detected in wild birds have not been completely characterized, including StHV 1, and therefore has not been approved as species and are marked as other related viruses which may be member of the family Herpesviridae
[13][14].
The clinical signs of herpesviruses infections in owls, known as inclusion body disease or herpesvirus hepatitis, appear as general depression and anorexia before death or sudden death
[15]. In addition, more specific clinical signs such as ulcerative superficial keratitis, proliferative conjunctivitis, and iris pigmentary changes have been described
[16]. Hepatitis and disseminated focal necrosis in the liver, spleen, and bone marrow are most commonly seen in owls dying of herpesvirus infections
[13]. The actual impact of herpesvirus on the wild owl population became apparent in cases of repopulation of eagle owls (
Bubo bubo) where serologically herpesvirus-negative birds released back to the wild led to the establishment and expansion of the population
[17]. Earlier studies of owl herpesvirus in Europe reported that, on the one hand, the virus can be lethal to some owl species but non-infective to some others, especially species with dark eyes (i.e., the tawny owl (
Strix aluco) and barn owl (
Tyto alba)
[18][19]), while the third European owl species with dark eyes, the Ural owl (
Strix uralensis), was not tested. A later study, which included Ural owls as well, did not confirm this hypothesis but indicated that polymorphic species are seemingly more resistant than the species showing lower variability in overall plumage color
[11]. However, all of these studies were conducted on dead or injured birds from wildlife rehabilitation centers or birds in laboratory experiments rather than in free-living wild populations.
The viral DNA polymerase is a key enzyme in the lytic phase of the infection by herpesviruses
[20]. PCR assays
[21][22] with degenerate primers for amplification of the herpesvirus DNA polymerase gene sequence have shown tremendous effectiveness in detecting previously unknown herpesvirus
[23]. Based on a partial herpesvirus DNA polymerase sequence phylogenetic study showed that herpesvirus in owls and herpesvirus endemic in the pigeon population—namely,
Columbid herpesvirus 1 (CoHV 1)—are the same virus, and that the pigeons are responsible for transmission of the virus to the owls
[7][24]. However, a recent study of phylogenetic analysis of herpesvirus DNA polymerase partial nucleotide sequences detected in dead owls showed that owls were also infected with herpesviruses that are divergent from CoHV 1
[11]. In general, inhalation of virus-containing dust derived from feathers, nasal excretion, saliva, nasal discharge, urine, feces, and crop milk is the predominant means of herpesvirus transmission in birds, and no vertical transmission has been proven
[25].
2. Herpesvirus Detection in Owls and Yellow-Necked Mouse Populations
The partial sequence of herpesvirus DNA polymerase gene was detected only in oropharyngeal swabs in 16 out of 170 owls examined (9.4%). However, the herpesvirus was detected only in Ural owls, in adults and young, but not in tawny owls (Table 1). Out of 45 Ural owls from Mount Krim, 14 were positive (31.1%), and out of 10 Ural owls from the Jelovica Plateau, 2 were positive (20.0%), but the infection prevalence was not significantly different between the areas (χ² = 0.11, p = 0.74). The infection prevalence was found to be higher in young than adult birds, although the difference was not significant (χ² = 0.59, p = 0.44). We found that about half of the Ural owl nests were infected (Table 1), but we did not necessarily confirm infection in all birds from each infected nest. The herpesvirus prevalence was found to be similar in adult females (22.2%, n = 9) and males (16.7%, n = 6). In the nests with more than one young, of which at least one was positive (n = 5 nests), the median herpesvirus prevalence in the young was 50.0% (range 20.0 to 100.0%). In the nests with tested breeding female and chicks, all chicks were infected when female was positive (n = 2 nests), while positive chicks were found also in the nests with females found negative for herpesvirus infection (n = 4 nests). In one nest in 2019 on Mount Krim, the Ural owl pair successfully raised one Ural and one tawny owl young, which was a consequence of competitive expulsion of the tawny owl by the larger Ural owl from the nestbox. Both Ural owl parents were herpesvirus positive as well as the Ural owl young, but not the tawny owl young raised in the same nest. All the owls sampled were clinically healthy, and the productivity and clutch and brood size between infected (median 4.0/3.5) and uninfected nests (median 3.5/3.0) was similar (Mann–Whitney U = 20–23, p = 0.6–1).
Table 1. Herpesvirus prevalence (% of infected among all tested birds/nests) in wild coexisting populations of tawny owls (Strix aluco) and Ural owls (Strix uralensis) in Slovenia in 2017 and 2019. The numbers of birds or nests examined are given in parentheses.
| Tawny Owl |
Ural Owl |
| Infected birds |
0.0% (115) |
29.1% (55) |
| Infected adults |
0.0% (27) |
18.7% (16) |
| Infected young |
0.0% (88) |
33.3% (39) |
| Infected nests |
0.0% (30) |
53.0% (17) |
In 2019, 40 yellow-necked mice captured at Mount Krim and the Jelovica Plateau were examined for the presence of herpesvirus. Pharyngeal and rectal swabs and tissue samples were analyzed with panherpesvirus PCR targeting the DNA polymerase gene. Of 40 free-living mice examined, 2 were herpesvirus positive (5%).
3. Phylogenetic and Sequence Analysis
The partial nucleotide herpesvirus sequences (205 nt) detected in wild Ural owls and yellow-necked mice were compared to the sequences of DNA polymerase gene of other avian and mammal herpesviruses to determine their phylogenetic relationship.
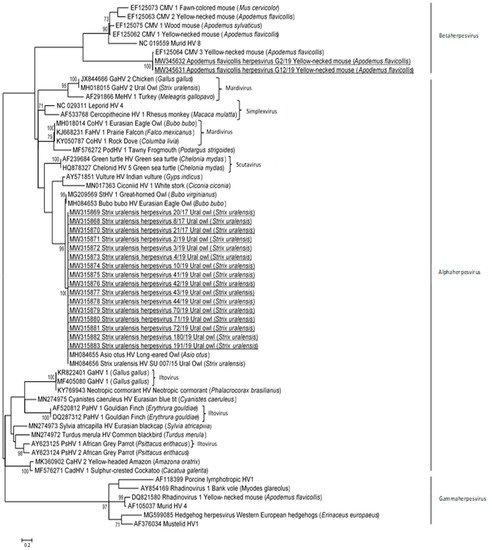
The phylogenetic analyses showed that the herpesvirus sequences detected in Ural owls are most closely related to the alphaherpesvirus sequences detected in other owls, whereas the herpesvirus sequences detected in small rodents formed a separate group among betaherpesviruses (Figure 1). More precisely, the sequences of herpesviruses detected in owls clustered together with novel herpesvirus sequences detected in the Eurasian eagle owl (Bubo bubo HV), Ural owl (Strix uralensis HV), long-eared owl (Asio otus HV), and great horned owl (StHV 1). The phylogenetic tree (Figure 1) showed that partial DNA polymerase gene sequences of wild Ural owls from Slovenia were identical and showed 100% nt identities with the herpesvirus sequence detected in the long-eared owl and Ural owl, and 90.6% and 90.0% nt identities with the Eurasian eagle owl and great horned owl, respectively. Known herpesvirus sequences detected in pigeons (CoHV 1) and birds of prey (CoHV 1, FaHV 1, GaHV 2) were grouped in the other clusters, and they shared 60.3% to 62.2% nt identities with novel herpesvirus sequences detected in Ural owls.
Figure 1. Phylogenetic relationship based on partial DNA polymerase gene nucleotide sequences of herpesviruses from Ural owls and yellow-necked mice captured in Slovenia and other herpesviruses derived from the GenBank database. The scale bar indicates substitutions per site. Nucleotide sequences obtained in the current study are additionally underlined.
The DNA polymerase sequences detected in yellow-necked mice were identical and shared 98.7% nt identity with the most closely related betaherpesvirus sequence detected in yellow-necked mice (Apodemus flavicollis cytomegalovirus 3) from Germany. Lower nt identities, 47.2 to 48.4%, were detected with other betaherpesvirus sequences in the yellow-necked mouse (Apodemus flavicollis cytomegalovirus 2 and 1), fawn-colored mouse (Mus cervicolor cytomegalovirus 1), and wood mouse (Apodemus sylvaticus cytomegalovirus 1).
There was low nt identity (51.1%) between the herpesvirus sequence detected in wild mice and owls in Slovenia.