1000/1000
Hot
Most Recent

Extracellular vesicles (EVs) are important players for intercellular communication. EVs are secreted by almost all cell types; they can transfer information between nearby or distant cells, and they are highly abundant in body fluids. The present work provides an overview of the components, effects, and applications of EVs in blood.
Extracellular vesicles (EVs) are membrane-coated particles secreted by almost all cell types. Their first identification was already reported in 1946 as procoagulant platelet-derived particles in normal plasma [1], and more than 20 years later, in 1967, they were referred as “platelet-dust” [2]. Since then, several publications started to report novel particle sources and functions and, by the end of the 20th century, they were already known to play a role in relevant processes, such as antigen presentation [3]. Importantly, at the beginning of the present century, research on EVs gained interest among the scientific community, as they were implicated in other central issues, such as the immune system mediated antitumour response [4], and also due to the discovery that EVs transfer mRNAs and microRNAs (miRNAs) from the donor to recipient cells inducing functional changes [5]. In the last two decades, thousands of works have continued to describe the characteristics, functions, and implications of EVs and their cargo in intercellular communication. Thanks to all of them, we can now state that EVs are important players in most biological processes.
EVs are present in diverse tissues and biofluids, and one of the most studied sources is blood. Circulating EVs are relatively easy to obtain with minimally invasive samplings, and more importantly, they have been found to be essential mediators of cell communication between different tissues and to be implicated in diverse cellular processes [6][7][8][9][10]. EVs originated from blood cells and from many other tissues compose the complex pool of EVs circulating in blood, and similarly, they can reach most of the tissues of the organism. Furthermore, due to the possibility of directing EVs in circulation to other tissues, the potential use of EV-based treatments through administration in blood has also been proposed and investigated. The aim of this review is to gather and present the studies of EVs that have been carried out in EVs isolated from blood or with EVs introduced in circulation, and to gain the whole perspective of EVs in circulation and their potential uses.
The term EVs is used to refer to all the particles that cells secrete to the extracellular media. There are two main categories of EVs: exosomes and microvesicles. Besides, apoptotic bodies are also considered EVs. Indeed, apoptotic bodies play an essential role in the proper clearance of the dying cell, as well as for the signalling of this programmed cell death to surrounding cells and for the regeneration of the tissue [11]. However, most of the works studying EVs are focused on exosomes and microvesicles, due to their multiple functions and implications. Exosomes are secreted particles originated from the fusion of a multivesicular body and the plasma membrane, while microvesicles are formed by the direct budding and fission of the plasma membrane.
With regard to the molecules carried by EVs, we have to consider both their membrane and inner cargo. The membrane of EVs consists mainly of proteins and lipids, but each EV has distinct types of proteins and lipids depending on their origin and function. Furthermore, the composition of the EV membrane influences the fate and internalization by recipient cells [12]. The components of the EV lumen are even more diverse and include proteins and many different nucleic acids. Apart from the above-mentioned mRNA and miRNAs, EVs carry other types of small and long non-coding RNAs, circular RNAs, and double-stranded DNA fragments [13][14][15]. Importantly, investigations about EV secretion and their cargo have revealed that the sorting of components into a forming particle is a controlled mechanism and not a random packaging of the available molecules in the secreting cell [5][16].
Similarly, the uptake of EVs is a controlled process. Many authors have studied the binding and internalization of EVs by recipient cells and multiple molecules, such as tetraspanins, integrins, lipids, and lectins, which have been identified to mediate the uptake [17]. The integration of EVs by recipient cells can be performed by the fusion of the EV and cellular membranes, or by distinct endocytic pathways. An extensive and complete review on the biogenesis, release, and targeting mechanisms of EVs was recently published, and it is a recommended read to go into this subject in depth [12].
EVs secreted by blood cells, but also EVs originated from many other tissues, can be found in the circulatory system. As a consequence, the studies performed in EVs isolated from blood samples have shown the complex pool of particles present in this fluid (Figure 1).
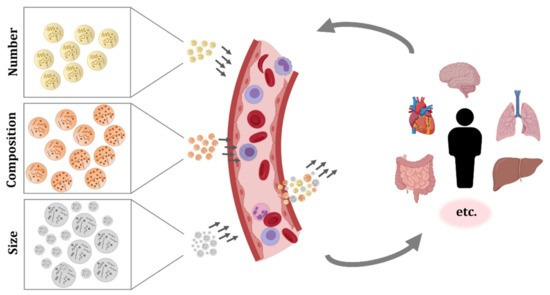
The most abundant EVs in blood are the ones from platelets. They were also the first ones to be described and were referred to as “platelet-dust” [1][2]. Investigations of EVs in plasma revealed the abundance of EVs from platelets as well as from erythrocytes, but also the influence of preanalytical factors such as blood sample storage time, temperature, and anticoagulants on their secretion [18][19][20].
Apart from platelets and erythrocytes, other blood cells also produce EVs. For instance, it has been demonstrated that cells such as mast cells, neutrophils, and eosinophils secrete EVs, but these reports are from in vitro experiments and they do not verify the presence of EVs from those cell types in circulation [21][22][23]. In the case of basophils, to our knowledge, there is no evidence of EV secretion. Under normal conditions, there are few basophils circulating in blood, and when activated, they secrete the contents of the high amount of granules they bear, releasing the inner content, but they do not produce EVs [24]. The production and secretion of EVs by other cell populations circulating in blood, including monocytes, macrophages, dendritic cells, NK cells, and B and T lymphocytes has been extensively reported, but similarly, most works were performed with cultured cells [3][25][26][27][28] and only few publications identified EVs in circulation with characteristic membrane molecules that could confirm the in vivo secretion of EVs from these immune cells [29][30][31][32]. Recently, a comprehensive work further evaluated the membrane molecules of EVs from plasma and serum, and pointed to the importance of the EV source and detection method on the identification of the markers [33].
One of the first works that identified EVs secreted by other cell types in blood was published more than 20 years ago. They detected vitronectin receptor (αvβ3) and other endothelial markers in human umbilical vein endothelial cells (HUVECs) and EVs secreted by these cells in vitro, and importantly, they also confirmed the presence of endothelial EVs in human plasma [34]. Similarly, EVs positive for adipocyte-specific markers such as adiponectin and resistin were first found in mice serum, and later, the presence of adipocyte-derived EVs in human plasma was confirmed by several adipocyte markers and adipokines [35][36][37][38]. The connection between the liver and the circulatory system suggested the presence of EVs from the liver in blood; this was confirmed in mice and human samples, in which hepatocyte-derived EVs were detected [39][40]. In addition, the muscle tissue also secretes EVs and, indeed, striated muscle-specific miRNAs or myo-miRNAs have been found in human plasma EVs [41][42]. Moreover, EVs produced by cardiomyocytes have been identified in mice and humans, as measured by cardiac bridging integrator 1 (cBIN1)-containing particles in circulation [43]. A systematic review on EVs secreted by six cardiac cell types, their cargo, and functions was published elsewhere, summarizing in vitro investigations and some studies performed in blood [44]. Another relevant communication system mediated by EVs was found in pregnant women, as indicated by the presence of placental-derived EVs (bearing placental-type alkaline phosphatase) in blood [45].
Notably, cancer cells from different origins also produce EVs, and these can enter the circulatory system [46][47][48]. Another type of EVs with great interest and potential uses was found in blood some years ago: EVs coming from the central nervous system (CNS). The ability of EVs to cross the blood–brain barrier (BBB) was confirmed and, thus, it is now known that particles produced by CNS cells can circulate in blood, and that EVs from diverse origins can enter the CNS [49][50][51].
Moreover, EVs are secreted in physiological and pathological conditions, and depending on their cargo and on the conditions of receptor cells, they can have beneficial or detrimental effects [52].
As introduced before, it is long known that EVs are functional particles. More than 60 years ago, EVs were described to play a role in blood coagulation [1][2]. Since then, EVs have been found to be secreted by almost all cell types and to play a role in diverse physiological processes.
For instance, one of the first studies that characterized the miRNA signature of circulating EVs in healthy donors found the angiogenesis-related miRNA-126 enriched in EVs [53]. In addition, circulating EVs have been found to influence immune response. A work carried out with human samples also found MCH-II, FasL, and other immune markers in plasma EVs, and besides, they reported an effect of circulating EVs on CD4 T cell response [54]. Another study showed that plasma EVs influence monocyte and B cell activation [29], and a work carried out in our group showed their influence on CD4 and CD8 T cell activation [55]. Therefore, it becomes evident that blood EVs have a role in immune system responses, but we are still far from being able to understand the complex mixture of circulating particles and their effects in the face of the numerous stimuli and insults that can occur to a single organism throughout time.
Regarding other physiological processes, EVs have been shown to be implicated in feto-maternal communication [45][56][57][58][59][60], in aging [55][61][62][63][64] and also to be affected by physical activity [65][66][67][68][69]. Similarly, EVs have been studied in many pathological processes, including neurological [70][71][72][73][74][75][76] and inflammatory diseases [77][78], vascular pathologies [79][80][81], infectious diseases [82][83][84][85], preganancy complications [86][87][88] or cancer [89][90][91][92][93][94][95] (Figure 2). Aiming to contribute to disease diagnosis, monitoring, and treatment, many researchers have investigated circulating EVs. Certainly, blood is a relatively easily accessible biofluid and can reflect modifications from different tissues and organs. However, much work is still required to validate the proposed candidates.
Figure 2. Extracellular vesicles (EVs) contribute to physiological and pathological processes. EVs secreted by different organs, tissues, and cell types reach systemic circulation. EV production and circulation occurs both in physiological and pathological states. Therefore, comparison of blood EVs from healthy and diseased donors, including different disease stages or treatments, is an interesting approach for the characterization of biological processes and biomarker discovery.
The use of cell therapies, particularly the ones based on stem cells, was proposed to have great potential. Additionally, in recent years, the possibility of administering EVs have gained interest, as they can have the same beneficial effects as progenitor cells while reducing their risks, such as uncontrolled proliferation or transplant rejection [96]. Furthermore, the use of EVs has additional benefits, including the easier storage and distribution, as well as the numerous possible routes of administration and modes of application [97]. Another advantage of EVs is that they are formed from cells and thus, biologically designed to be taken up by recipient cells. In contrast, other constructs, such as liposomes or nanoparticles, are easily loaded with the molecule or drug of interest, but they could face biodistribution or targeting problems, reducing their efficacy [98]. Moreover, there are several techniques for obtaining EVs enriched in a particular compound. On the one side, we can modify the EV-producing cell by transfection or transduction by culturing them under a particular stress or condition, or by incubating them with the molecule of interest. On the other side, EVs can be modified after their production, by electroporation, sonication, extrusion, or other methods [98][99][100] (Figure 3).
Figure 3. Schematic representation of possible workflows for extracellular vesicle (EV) administration. EVs or producing cells can be isolated from human donors or other organisms. In the case of cells, specific culture protocols are applied to induce the production of EVs with the desired features or cargo. Therefore, EVs produced in culture are more homogeneous than EVs isolated from whole organisms. In any case, once EVs are isolated, other procedures can be applied to EVs, including enrichment, or loading of molecules of interest. Then, EVs are injected in circulation and, thanks to their membrane composition and cargo, they will migrate to the target tissue and exert their effect.
Many studies have been performed with EVs introduced in circulation, most of them in mice. Some investigations focused on the effect of EVs from patients with diverse pathologies, while others evaluated the impact of murine EVs. There are also some works that evaluated parasite-derived EVs [101][102][103]. A pioneering work in the field was published already 10 years ago, with dendritic cell-derived EVs. Primary cells from murine bone marrow were cultured and transfected to produce EVs that bear the CNS-targeting rabies viral glycoprotein (RVG) peptide at their membrane. Then, EVs were isolated and electroporated with the siRNA of interest. The intravenous injection of these EVs resulted in brain targeting and importantly, downregulation of BACE1—a protease implicated in amyloid aggregation in Alzheimer’s disease—was achieved [104]. Besides, this system was effectively applied to target Parkinson’s disease related to α-synuclein [105]. The effect of systemically administered EVs in the CNS has also been demonstrated in acute problems, for instance in traumatic brain injury. A single dose of miRNA-124-enriched EVs 24 h after traumatic brain injury in rats resulted in a significant improvement [106].
Moreover, the effect of systemic injection of EVs has been reported to also affect metabolic processes, such as insulin sensitivity [107][108]. The potential applications of EVs are also being investigated with regard to other pathologies and complications. In the case of transplantations, EVs enhanced allograft tolerance and long-term survival in animal models [109][110] and experiments performed with human EVs obtained promising results in a wound-healing mouse model [111]. In addition, a recent study points to the theranostic potential of autologous circulating EVs. The authors demonstrated that fluorescently labelled EVs from colorectal cancer patients, when injected in mice, selectively target patient-derived xenografts. Furthermore, their preliminary experiments performed in mast cells and mammary tumour-bearing dogs also showed tumour tropism of autologous plasma EVs that were fluorescently labelled [112].
These and other studies demonstrate the promising applicability of EVs in blood, but they also highlight the multiple approaches that are being tested and the need for standardization. Before routine clinical application of EVs could be reached, aspects such as the cell of origin, EV production and isolation methods, possible EV loading or modifications, storage, administration, and dosing should be further investigated [113][114][115].
Despite current knowledge limitations, there are already hundreds of clinical trials registered (clinicaltrials.gov) involving EVs, also termed exosomes or microparticles/microvesicles. It should be noted that most of them focus on the analysis of EVs from body fluids as biomarkers for disease diagnosis or monitoring. However, there are also clinical trials evaluating EV administration, and a high proportion of them test EVs introduced in circulation [116][117]. To date, there are few finished clinical trials that reported their results, but the available information indicates that EV administration is safe and well tolerated by patients with diverse pathologies. However, the efficacy of tested EV therapies is moderated. Results from ongoing and future clinical trials are needed to be able to critically evaluate the impact of EV-based treatments.