Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shusong Wu | + 2404 word(s) | 2404 | 2021-08-04 04:54:49 | | | |
| 2 | Vivi Li | Meta information modification | 2404 | 2021-08-13 09:44:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wu, S.; He, J. Polyphenols and Heat Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/13139 (accessed on 08 February 2026).
Wu S, He J. Polyphenols and Heat Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/13139. Accessed February 08, 2026.
Wu, Shusong, Jianhua He. "Polyphenols and Heat Stress" Encyclopedia, https://encyclopedia.pub/entry/13139 (accessed February 08, 2026).
Wu, S., & He, J. (2021, August 13). Polyphenols and Heat Stress. In Encyclopedia. https://encyclopedia.pub/entry/13139
Wu, Shusong and Jianhua He. "Polyphenols and Heat Stress." Encyclopedia. Web. 13 August, 2021.
Copy Citation
Heat stress is a non-specific physiological response of the body when exposed to high ambient temperatures, which can break the balance of body redox and result in oxidative stress that affects growth performance as well as the health of animal and poultry species. Polyphenols have attracted much attention in recent years due to their antioxidant ability and thus, can be an effective attenuator of heat stress.
heat stress
oxidative stress
polyphenols
poultry
1. Introduction
Heat stress is a stress response that can lead to various harmful impacts on livestock productivity, such as high animal morbidity, mortality, and reduction in growth performance, directly resulting in dramatic economic losses to the livestock industry. Global warming has created a massive challenge for the livestock industry, especially in tropical and subtropical zones which contribute most to global livestock production [1]. Compared with other animals, poultry is more sensitive to heat stress, which weakens their immunological functions and makes them more susceptible to infection by pathogens, leading to a decline in growth performance and in many cases, death [2][3][4]. Furthermore, heat stress may affect meat color and pH and is recognized as one of the primary influencing factors of meat quality [5][6][7]. Generally, growth performance decline caused by heat stress is directly related to a reduction in feed intake, but increasing evidence has shown that heat stress may induce reactive oxygen species (ROS) and cause anti-oxidant system disorders, which affect nutrient absorption and metabolism [8][9]. The crosstalk between heat stress and oxidant stress, as well as the effects and mechanisms of polyphenols on stress are reviewed in the following sections.
2. Heat Stress in Poultry
Poultry has no sweat glands, and respiratory hyperventilation is poultry’s primary mechanism of dissipating heat [10]. The optimum temperature for most poultry species is from 18 °C to 20 °C. However, when the temperature is above this range, breathing frequency will increase three-fold to overcome the heat stress [1][11]. Most poultry species will experience heat stress when the temperature rises above 32 °C, accompanied by physiological and metabolic disorders [12].
Multiple studies have shown that heat stress can significantly reduce feed intake, daily gain, and feed utilization [13]. Due to poultry’s feathers which cover their body and lack of sweat glands, a continuous high temperature will inhibit energy metabolism [10][14]. Poultry feed intake declines 1.5% for each degree rise in temperature when the temperature ranges from 21 °C to 30 °C, and the decline in feed intake will increase to 4.6% with a temperature range of 32 °C to 38 °C [15]. When poultry are exposed to heat stress, their body temperature, blood circulation, and peripheral blood flow increases sharply, whereas their visceral blood flow decreases. These changes lead to limited nutrients utilization and thus reduce the poultry’s production performance and feed conversion efficiency [16][17]. There is a significant negative correlation between feed intake and environmental temperature [15]. The effect of heat stress on feed intake in poultry is a complex process. On one hand, heat stress decreases the gastrointestinal motility and prolongs gastric emptying, which in turn results in lowered feed intake [18]. On the other hand, poultry will increase their water intake under heat stress and thus reduce the concentration of digestive enzymes in the intestinal tract, which can affect food digestion and absorption [19].
It is reported that heat stress negatively affects the laying performance and egg quantity of poultry. Exposure of laying hens to high temperatures resulted in a significant decrease in egg weight, shell weight, and shell thickness [20][21][22][23]. Due to metabolic differences and higher heat production by laying hens than broilers, heat stress has more significant impact on laying hens than on broilers. In a heat stress experiment looking at hens laying eggs, Mashaly et al. [17] reported that the weights of eggs in the heat stress group (35 °C and 15% relative humidity, 4 h/d) were significantly lower than in the control group throughout the five week experiment. Zhu et al. [22] reported that exposure to 32 °C could significantly decrease egg weight, laying rate, egg yield, and eggshell quality.
3. Potential Mechanisms Underlying Attenuation of Oxidative Stress by Polyphenols in Heat Stressed Poultry
Phytochemicals with antioxidant activity offer great hope as a solution for heat stress in poultry. As one of the critical secondary metabolic substances, polyphenols widely exist in a variety of plants and have been used for various purposes because of their strong antioxidant ability [24]. Polyphenols are characterized by phenol as the basic skeleton, and polyhydroxy substitution of the benzene ring can be classified into phenolic acids, acetophenones, phenylacetic acid, hydroxycinnamic acids, coumarins, naphthoquinones, xanthones, stilbenes, and flavonoids [24]. The following sections summarize the potential mechanisms underlying the antioxidant functions of three common polyphenols (as shown in Figure 1) based on our previous studies and recent peer-reviewed studies.
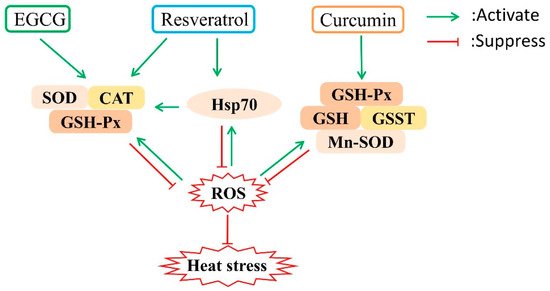
Figure 1. Potential mechanisms underlying the protective effect of polyphenols against heat stress. Polyphenols can upregulate the expression of stress response proteins such as heat shock proteins and antioxidant enzymes, which can suppress reactive oxygen species (ROS). EGCG, epigallocatechin gallate; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; Hsp70, heat shock proteins 70; GSH, glutathione; GSST, glutathione S-transferase; Mn-SOD, manganese superoxide dismutase.
3.1. Resveratrol
Resveratrol, 3,5,4’-trihydroxystilbene, is a kind of natural plant antitoxin, which belongs to the category of non-flavonoid polysaccharides and is also an antibiotic secreted by plants to resist fungal infection under pathogen attack. Resveratrol exhibits lipophilic characteristics which lead to high absorption, but the systemic bioavailability of resveratrol is relatively low. The plasmatic concentration of resveratrol plus total metabolites is around 400–500 ng/mL (≈2 μM) after a 25 mg oral dose [25]. An isotope tracer experiment has shown that radioactivity derived from 14C-resveratrol can be detected in various organs such as the liver, kidney, brain, heart, lung, testis, and intestine after oral intake of 14C-resveratrol. This suggests that resveratrol or its metabolites can enter almost all organs after intake [26]. Resveratrol shows a strong antioxidant ability in poultry. Sahin et al. [27] reported that quails supplemented with 400 mg/kg resveratrol had a lower serum MDA concentration (p < 0.05) and a higher serum vitamin E concentration (p < 0.05). Zhang et al. [28] revealed that supplementation of 400 mg/kg resveratrol increased muscle glycogen content and the activities of total superoxide dismutase (T-SOD) and GSH-Px (p < 0.05) but decreased muscle MDA content and lactate dehydrogenase (LDH) activity (p < 0.05) in transport stress-impaired broilers. Liu et al. [29] also reported that supplementation of 200, 400, or 600 mg/kg resveratrol efficiently attenuated heat stress in black-boned chickens, and the dose of 400 mg/kg showed the strongest antioxidant effect.
Many studies have reported that resveratrol can effectively scavenge ROS, regulate the activity of various antioxidant enzymes, reduce DNA damage, and significantly enhance the expression levels of various antioxidant enzymes and proteins. The antioxidant effect of resveratrol is stronger than that of vitamin C, and it is more effective in scavenging ·OH. Das et al. [30] reported that dietary supplementation of resveratrol can inhibit lipid peroxidation and improve enzyme (SOD, GSH-Px, CAT) activity (p < 0.05) in hepatocytes, thus relieving liver damage caused by heat stress in rats. Zhang et al. [31] also reported that resveratrol could protect against the heat stress-impaired meat quality of broilers by increasing the muscle total antioxidant capacity (T-AOC) and activity of antioxidant enzymes (CAT, GSH-PX). Our previous study revealed that resveratrol could significantly increase the average daily feed intake (ADFI) and average daily weight gain (ADG) of black-boned chickens and reduce feed to gain ratio (F/G ratio) under the condition of heat stress. This occurred as a result of the restoration of antioxidant enzyme activity that was decreased by heat stress [29][32]. Liu et al. [33] reported that resveratrol could alleviate intestinal injuries by increasing mRNA and protein expression of Hsp70, Hsp90, and NF-kappa B, and suppressing the production of epidermal growth factor (EGF) in the mucosa.
3.2. Curcumin
Curcumin (feruloyl methane) is a yellow polyphenol extracted from the traditional Chinese medicine, zingiber plant. Since the discovery of curcumin in 1815, it has become one of the most widely used natural pigments. Animals can easily absorb curcumin. Ravindranath et al. [34] reported that about 60% was absorbed after oral administration of 400 mg curcumin to rats. In another rat experiment conducted by Marczylo and colleagues [35], curcumin was found in the plasma (16.1 ng/mL), urine (2.0 ng/mL), intestinal mucosa (1.4 mg/g), liver (3671.8 ng/g), kidney (206.8 ng/g), and heart (807.6 ng/g) after oral intake of 340 mg/kg of curcumin. Curcumin can therefore effectively distribute to all parts of the body after intake, potentially undergo metabolic O-conjugation to curcumin glucuronide and curcumin sulfate, as well as bio-reduction to tetrahydrocurcumin, hexahydrocurcumin, octahydrocurcumin, and hexahy-drocurcuminol [36].
The 1,1-diphenyl-2-picryl-hydrazyl free radical (DPPH) and 2,2′-azino-bis (3-ethylbenzthiazoline -6-sulfonic acid) (ABTS) radical scavenging ability of curcumin is higher than that of artificial antioxidant butylated hydroxyanisole (BHA) [37]. Many studies have shown that curcumin can improve poultry’s growth performance under heat stress [38][39][40]. Curcumin can restore the impaired growth performance caused by heat stress potentially due to its capacity to mitigate the broilers’ mitochondrial dysfunction and enhance the mitochondrial biogenesis caused by heat stress. Zhang et al. [40] reported that curcumin decreased the ROS production of broilers after heat stress by increasing the mitochondrial Mn-SOD activity and gene expression of thioredoxin 2 and peroxiredoxin-3. Curcumin can also decrease the mitochondrial malondialdehyde levels and increase mitochondrial glutathione content as well as the activities of GSH-Px, glutathione S-transferase (GSST), and Mn-SOD [39].
3.3. Epigallocatechin Gallatel
Epigallocatechin gallate (EGCG) is the primary component of green tea extract, which possesses strong antioxidant and anti-inflammatory properties and shows higher bioavailability than other polyphenols. The absolute bioavailability of EGCG in mice is 26.5% according to Lambert et al. [41]. Epigallocatechin gallate undergoes methylation, glucuronidation, and sulfation in vivo [41], but mostly presents in free form in the plasma and can thus effectively spread around the body [42]. Suganuma et al. [43] directly administered the [H-3] (−)-epigallocatechin gallate ([H-3]EGCG) into the mouse stomach and found significant radioactivity in the digestive tract, liver, lung, pancreas, mammary gland, skin, brain, kidney, uterus ovary, and testes. Multiple studies have reported that EGCG can attenuate heat stress in poultry. Xue et al. [44] revealed that supplementation of 300 and 600 mg/kg EGCG in heat-stressed broilers could increase growth performance in a dose-dependent manner. Luo et al. [45] also reported that a supplement of 600 mg/kg EGCG in heat-stressed broilers showed the best antioxidant property. Sahin et al. [46] reported that supplement with 400 mg/kg EGCG in heat-stressed quails showed the best antioxidant property. Thus 400–600 mg/kg of EGCG may be the optimal dosage in poultry.
Supplemental EGCG can maintain the equilibrium of oxidation–reduction and improve the expression of antioxidant genes (SOD, CAT, GSH-Px), thus reducing the damage caused by oxidative stress [47]. Supplemental EGCG can improve the poultry’s growth performance and alleviate the oxidant damage under heat stress by modulating the antioxidant enzymes [44][45][46]. Epigallocatechin gallate enhances antioxidant enzyme activity mainly due to the fact that it can activate nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathways. Sahin et al. [46] reported that EGCG improves antioxidant capacity by regulating the transcription factor Nrf2 as well as Nrf2-regulated heme oxygenase -1(HO-1). However, Orhan et al. [48] reported that EGCG could also decrease hepatic expression of activator protein-1 (AP-1), cyclooxygenase-2 (COX-2), and heat shock proteins (Hsps). Thus, EGCG potentially attenuates heat stress through modulating stress-responsive transcription factors.
The beneficial effects of polyphenols as a potential attenuator of heat stress are mainly attributed to their antioxidant activities. Polyphenols can act as radical scavengers depending on their chemical structures [49] and thus scavenge ROS including O2•−, •OH, and H2O2 to eliminate the oxidative damage caused by heat stress [50]. The phenol functional group of polyphenols can donate a hydrogen atom to the free radicals and direct quenching ROS. Also, they may block the action of some enzymes (e.g. xanthine oxidase and protein kinase C) that directly generate O2·− [51][52]. Furthermore, polyphenols can modulate cell signaling pathways to alleviate the impact of heat stress. On the one hand, resveratrol [33], EGCG [46], and curcumin [36] have been proven as potent inhibitors of nuclear factor kappa B(NF-κB), which can transcribe inflammatory markers such as interleukin-6 (IL-6), interleukin-2 (IL-2), and tumor necrosis factor α (TNF-α) to cause inflammation [53]. On the other hand, resveratrol [54], EGCG [46], and curcumin [38] may upregulate the antioxidant response pathways such as the transcription factor Nrf2 mediated antioxidant enzymes to improve the antioxidant enzyme system. Under a heat stress situation, resveratrol and EGCG can enhance the activity of antioxidant enzymes (CAT, GSH-Px, SOD) [31][32] and non-enzyme systems (GSH [32], vitamin E [27]), while curcumin can increase the activity of Mn-SOD, GSH-Px, and GSST [38]. In addition, polyphenols may attenuate heat stress by regulating the expression of heat shock proteins. Resveratrol can upregulate the transcription level of Hsp70 and Hsp90 in the immune organs of black-boned chickens under heat stress and directly participate in the immune response against the damage caused by heat stress [29]. However, EGCG has been reported to suppress the elevated hepatic expression of Hsps caused by heat stress, specifically inhibiting the expression of Hsp70 and Hsp90 by restraining the promoter activity [48]. To our knowledge, there is no direct evidence to demonstrate the mechanism of polyphenols alleviating damage caused by heat stress through the inhibition of Hsps expression.
The bioavailability of polyphenols remains controversial due to the low absorption rate since polyphenols undergo various metabolisms such as methylation, glucuronidation, and sulfation after intake [41]. Generally, the concentration of the parent structure of polyphenols rather than their metabolites were detected in early studies [55]. However, recent studies have shown that phenolic metabolites possess strong biological activities, thus the bioavailability of polyphenols should not exclude their phenolic metabolites [56]. The stomach and small intestine are the main absorption sites of polyphenols. An isotope tracer experiment with resveratrol showed that the concentration of radioactivity in the intestinal tract was the highest [26]. The concentration of curcumin in intestinal mucosa can reach 1.4 mg/g after intake of 340 mg/kg curcumin [35]. Around 33% of EGCG can be excreted in feces [42]. Therefore, the gut should be the main place for the function of polyphenols. The liver is the main metabolic organ and also a target of polyphenols. The concentration of curcumin in the liver can reach 3.6 mg/g at a dose of 340 mg/kg [35]. High concentrations of radioactivity were detected in the liver in the isotope tracer experiment with resveratrol and EGCG [26][42]. The high concentration of polyphenols in the liver can potentially explain the hepatoprotection ability under heat stress. In addition, polyphenols can also reach circulation and other organs. Plasma concentration of curcumin can reach 16.1 ng/mL at a dose of 340 mg/kg [35]. Radioactivity can be detected in the kidney, testis, lung, pancreas, skin, mammary gland, and even brain after intake of isotope labeled resveratrol and EGCG [25][43]. This may also explain why polyphenols are capable of attenuating systemic disorders caused by heat stress.
References
- Renaudeau, D.; Collin, A.; Yahav, S.; de Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728.
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sa, L.R.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Green Version]
- Lin, H.; Zhang, H.F.; Du, R.; Gu, X.H.; Zhang, Z.Y.; Buyse, J.; Decuypere, E. Thermoregulation responses of broiler chickens to humidity at different ambient temperatures. II. Four weeks of age. Poult. Sci. 2005, 84, 1173–1178. [Green Version]
- Quinteiro-Filho, W.M.; Gomes, A.V.; Pinheiro, M.L.; Ribeiro, A.; Ferraz-de-Paula, V.; Astolfi-Ferreira, C.S.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012, 41, 421–427. [Green Version]
- Wang, R.H.; Liang, R.R.; Lin, H.; Zhu, L.X.; Zhang, Y.M.; Mao, Y.W.; Dong, P.C.; Niu, L.B.; Zhang, M.H.; Luo, X. Effect of acute heat stress and slaughter processing on poultry meat quality and postmortem carbohydrate metabolism. Poult. Sci. 2017, 96, 738–746.
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Green Version]
- McKee, S.R.; Sams, A.R. The effect of seasonal heat stress on rigor development and the incidence of pale, exudative turkey meat. Poult. Sci. 1997, 76, 1616–1620.
- Quinteiro, W.M.; Rodrigues, M.V.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sa, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: Role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 2012, 90, 1986–1994.
- Yang, L.; Tan, G.Y.; Fu, Y.Q.; Feng, J.H.; Zhang, M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2010, 151, 204–208.
- Tesseraud, S.; Temim, S. Modifications métaboliques chez le poulet de chair en climat chaud: Conséquences nutritionnelles. Prod. Anim. 1999, 12, 353–363.
- Farag, M.R.; Alagawany, M. Physiological alterations of poultry to the high environmental temperature. J. Biol. 2018, 76, 101–106.
- Seven, P.T.; Seven, I.; Yilmaz, M.; Simsek, U.G. The effects of Turkish propolis on growth and carcass characteristics in broilers under heat stress. Anim. Feed Sci. Technol. 2008, 146, 137–148.
- He, S.J.; Zhao, S.J.; Dai, S.F.; Liu, D.Y.; Bokhari, S.G. Effects of dietary betaine on growth performance, fat deposition and serum lipids in broilers subjected to chronic heat stress. Anim. Sci. J. 2015, 86, 897–903.
- Pech-Waffenschmidt, V.; Bogin, E.; Avidar, Y.; Horst, P. Metabolic and biochemical changes during heat stress in relation to the feathering degree of the domestic hen. Avian Pathol. 1995, 24, 33–44. [Green Version]
- Payne, C.G. Practical Aspects of Environmental Temperature for Laying Hens. World Poult. Sci. J. 1966, 22, 126–139.
- Dai, S.F.; Wang, L.K.; Wen, A.Y.; Wang, L.X.; Jin, G.M. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br. Poult. Sci. 2009, 50, 333–340.
- Mashaly, M.M.; Hendricks, G.L., 3rd; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894.
- Datta, U.K. Effect of heat stress on gastro-intestinal motility in young albino rats. Indian J. Physiol. Pharmacol. 2001, 45, 222–226.
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Green Version]
- Torki, M.; Zangeneh, S.; Habibian, M. Performance, egg quality traits, and serum metabolite concentrations of laying hens affected by dietary supplemental chromium picolinate and vitamin C under a heat-stress condition. Biol. Trace Elem. Res. 2014, 157, 120–129.
- Kilic, I.; Simsek, E. The Effects of Heat Stress on Egg Production and Quality of Laying Hens. J. Anim. Vet. Adv. 2013, 12, 42–47.
- Zhu, Y.W.; Xie, J.J.; Li, W.X.; Lu, L.; Zhang, L.Y.; Ji, C.; Lin, X.; Liu, H.C.; Odle, J.; Luo, X.G. Effects of environmental temperature and dietary manganese on egg production performance, egg quality, and some plasma biochemical traits of broiler breeders. J. Anim. Sci. 2015, 93, 3431–3440.
- Karami, M.; Torki, M.; Mohammadi, H. Effects of dietary supplemental chromium methionine, zinc oxide, and ascorbic acid on performance, egg quality traits, and blood parameters of laying hens subjected to heat stress. J. Appl. Anim. Res. 2018, 46, 1174–1184.
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043.
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382.
- Vitrac, X.; Desmouliere, A.; Brouillaud, B.; Krisa, S.; Deffieux, G.; Barthe, N.; Rosenbaum, J.; Merillon, J.M. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003, 72, 2219–2233.
- Sahin, K.; Akdemir, F.; Orhan, C.; Tuzcu, M.; Hayirli, A.; Sahin, N. Effects of dietary resveratrol supplementation on egg production and antioxidant status. Poult. Sci. 2010, 89, 1190–1198. [Green Version]
- Zhang, C.; Wang, L.; Yang, L.; Zhao, X.H.; Chen, X.Y.; Geng, Z.Y. Dietary resveratrol supplementation prevents transport-stress-impaired meat quality of broilers through maintaining muscle energy metabolism and antioxidant status. Poult. Sci. 2017, 96, 2219–2225.
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62.
- Das, S.; Tosaki, A.; Bagchi, D.; Maulik, N.; Das, D.K. Potentiation of a survival signal in the ischemic heart by resveratrol through p38 mitogen-activated protein kinase/mitogen- and stress-activated protein kinase 1/cAMP response element-binding protein signaling. J. Pharmacol. Exp. Ther. 2006, 317, 980–988.
- Zhang, C.; Zhao, X.; Wang, L.; Yang, L.; Chen, X.; Geng, Z. Resveratrol beneficially affects meat quality of heat-stressed broilers which is associated with changes in muscle antioxidant status. Anim. Sci. J. 2017, 88, 1569–1574.
- He, S.; Li, S.; Arowolo, M.A.; Yu, Q.; Chen, F.; Hu, R.; He, J. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim. Sci. J. 2019, 90, 401–411.
- Liu, L.L.; Fu, C.X.; Yan, M.L.; Xie, H.B.; Li, S.; Yu, Q.F.; He, S.P.; He, J.H. Resveratrol modulates intestinal morphology and HSP70/90, NF-kappa B and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016, 7, 1329–1338.
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265.
- Marczylo, T.H.; Steward, W.P.; Gescher, A.J. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J. Agric. Food Chem. 2009, 57, 797–803.
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18.
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37.
- Zhang, J.F.; Bai, K.W.; Su, W.P.; Wang, A.A.; Zhang, L.L.; Huang, K.H.; Wang, T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018, 97, 1209–1219. [Green Version]
- Zhang, J.F.; Hu, Z.P.; Lu, C.H.; Yang, M.X.; Zhang, L.L.; Wang, T. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 2015, 93, 1656–1665.
- Zhang, J.; Bai, K.W.; He, J.; Niu, Y.; Lu, Y.; Zhang, L.; Wang, T. Curcumin attenuates hepatic mitochondrial dysfunction through the maintenance of thiol pool, inhibition of mtDNA damage, and stimulation of the mitochondrial thioredoxin system in heat-stressed broilers. J. Anim. Sci. 2018, 96, 867–879. [Green Version]
- Lambert, J.D.; Lee, M.J.; Lu, H.; Meng, X.F.; Ju, J.; Hong, J.; Seril, D.N.; Sturgill, M.G.; Yang, C.S. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003, 133, 4172–4177.
- Lee, M.J.; Maliakal, P.; Chen, L.S.; Meng, X.F.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032.
- Suganuma, M.; Okabe, S.; Oniyama, M.; Tada, Y.; Ito, H.; Fujiki, H. Wide distribution of H-3 (−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 1998, 19, 1771–1776.
- Xue, B.; Song, J.; Liu, L.; Luo, J.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and antioxidant capacity in heat-stressed broilers. Arch. Anim. Nutr. 2017, 71, 362–372.
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018, 97, 599–606.
- Sahin, K.; Orhan, C.; Tuzcu, M.; Ali, S.; Sahin, N.; Hayirli, A. Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult. Sci. 2010, 89, 2251–2258. [Green Version]
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99.
- Orhan, C.; Tuzcu, M.; Gencoglu, H.; Sahin, N.; Hayirli, A.; Sahin, K. Epigallocatechin-3-gallate exerts protective effects against heat stress through modulating stress-responsive transcription factors in poultry. Br. Poult. Sci. 2013, 54, 447–453.
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584.
- Neudorffer, A.; Desvergne, J.P.; Bonnefont-Rousselot, D.; Legrand, A.; Fleury, M.B.; Largeron, M. Protective effects of 4-hydroxycinnamic ethyl ester derivatives and related dehydrodimers against oxidation of LDL: Radical scavengers or metal chelators? J. Agric. Food Chem. 2006, 54, 1898–1905.
- Kurek-Gorecka, A.; Rzepecka-Stojko, A.; Gorecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2014, 19, 78–101.
- Levites, Y.; Amit, T.; Youdim, M.B.H.; Mandel, S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J. Biol. Chem. 2002, 277, 30574–30580.
- Sahin, K.; Orhan, C.; Smith, M.O.; Sahin, N. Molecular targets of dietary phytochemicals for the alleviation of heat stress in poultry. World Poult. Sci. J. 2013, 69, 113–124.
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s.
- Wu, S.; Hu, R.; Nakano, H.; Chen, K.; Liu, M.; He, X.; Zhang, H.; He, J.; Hou, D.-X. Modulation of Gut Microbiota by Lonicera caerulea L. Berry Polyphenols in a Mouse Model of Fatty Liver Induced by High Fat Diet. Molecules 2018, 23, 3213.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
13 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




