1000/1000
Hot
Most Recent

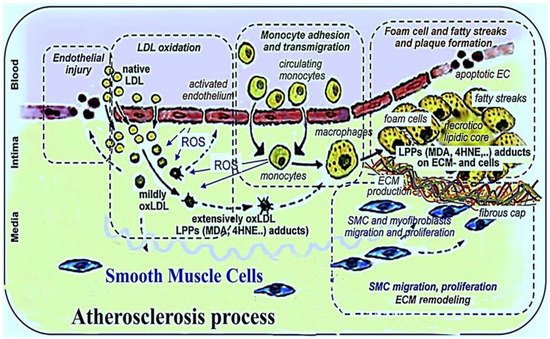
Atherosclerosis is a chronic inflammatory disease which is a major cause of coronary heart disease and stroke in humans. It is characterized by intimal plaques and cholesterol accumulation in arterial walls. The side effects of currently prescribed synthetic drugs and their high cost in the treatment of atherosclerosis has prompted the use of alternative herbal medicines, dietary supplements, and antioxidants associated with fewer adverse effects for the treatment of atherosclerosis. Natural and synthetic antioxidants have a crucial role in the prevention and treatment of atherosclerosis through different mechanisms. These include: The inhibition of low density lipoprotein (LDL) oxidation, the reduction of reactive oxygen species (ROS) generation, the inhibition of cytokine secretion, the prevention of atherosclerotic plaque formation and platelet aggregation, the preclusion of mononuclear cell infiltration, the improvement of endothelial dysfunction and vasodilation, the augmentation of nitric oxide (NO) bioavailability, the modulation of the expression of adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) on endothelial cells, and the suppression of foam cell formation.
Vitamin E is the most comprehensively studied lipid soluble antioxidant in humans. It consists of eight isomeric compounds (α-, β-, γ-, and δ- tocopherol; and α-, β-, γ-, and δ-tocotrienol) [9][66]. Cooking oils, egg yolk, butter, green leafy vegetables, and some fruit (kiwi fruit, pumpkins, mangoes, papayas, and tomatoes) are rich sources of vitamin E [9].
In several animal models, vitamin E has preventative effects against atherosclerosis by: Scavenging free radicals in VSMC, diminishing the oxidation of LDL by the inhibition of Cluster Differentiating 36 (CD36) and Scavenger receptor class B type I (SR-BI) expression in VSMC, reducing VSMC proliferation via the inhibition of protein kinase C (PKC), preventing foam cell formation, lessening the secretion of cytokines and extracellular matrix in VSMC, preventing mononuclear cell infiltration, lessening inflammation, curtailing the destabilization of fibrous plaque, inhibiting the apoptosis of VSMC, modulating signal transduction and gene expression in VSMC, increasing the expression of connective tissue growth factor (CTGF) in VSMC (cell lines), preventing endothelial dysfunction related to cholesterol, modulating endothelial cells and the expression of adhesion molecules such as VCAM-1 and ICAM-1 on endothelial cells, preventing lysophosphatidylcholine (LPC)-induced endothelial dysfunction and the preservation of endothelial NO release, modulating monocytes, macrophages, T cells and mast cells, enhancing the expression of cytosolic phospholipase A2 (PLA2), cyclooxygenase, and vasodilating prostacyclin (PGI2) in endothelial cells, inhibiting thrombin formation, and reducing leukotriene synthesis [67][68][69][70][71][72][73][74][75][76].
In several clinical studies, vitamin E revealed contrasting findings. In a study examining the effect of 50 mg·day-1 synthetic vitamin E in a population with coronary heart disease, the results showed no effect on major cardiovascular events [77]. Another study showed 300 mg day-1 synthetic vitamin E had no effect on cardiovascular disease, including the rate of non-fatal myocardial infarction in patients with previous myocardial infarction [78]. Additionally, vitamin E did not significantly decrease the incidence of cardiovascular disease such as stroke [79]. In the Cambridge heart antioxidant study (CHAOS), vitamin E supplementation failed to have an impact on cardiovascular outcomes in patients at high risk of cardiovascular events [80].
Vitamin C (ascorbate) is a water-soluble and ubiquitous antioxidant [7][23] with an ability to scavenge peroxyl radicals and HOCL [23][66], thus providing stability to the cell membrane. Fruit and vegetables, particularly citrus fruit, kiwi, cantaloupe, mango, strawberries, and peppers are rich sources of vitamin C [9]. It has various functions including: The improvement of nitric oxide-dependent vasomotor function [81], the enhancement of NOS activity (NO production) and the consequent augmentation of NO bioavailability, the improvement of endothelial dysfunction and vasodilation, the inhibition of cyclooxygenase, the diminishing of cell–cell adhesion [82], and the reduction of the chain-carrying α-tocopheroxyl radical to inhibit LDL peroxidation [83]. It also recycles other endogenous antioxidants, such as vitamin E [84]; discourages leukocyte aggregation and adhesion to the endothelium [9]; and scavenges ROS such as superoxide, hydroxyl radicals, peroxyl radicals, and many non-radicals, such as nitrosating agents and hydrochlorous acid [9].
A number of small-scale clinical studies have evaluated the effect of vitamin C on vascular health. The British Regional Heart Study demonstrated an inverse relation between plasma vitamin C concentration and endothelial dysfunction in men with no history of cardiovascular disease or diabetes [85]. Additionally, the European prospective investigation into cancer and nutrition (EPIC) Norfolk study showed the same results as the British Regional Heart Study in both men and women [86].
A large-scale study conducted over 20 years found that diets rich in vitamin C had no significant association with coronary heart disease [87].
B vitamins have a fundamental role in the metabolism of essential amino acids, with a specific influence on homocysteine and the antioxidant, glutathione [9]. Other significant activities of B vitamins entail scavenging hydroxyl and lipid peroxyl radicals, improving endothelial function, and ameliorating the coupling of endothelial NO synthase through the essential cofactor, tetrahydrobiopterin [88][89].
In a clinical study intake of folate, hydroxocobalamin, and pyridoxine, supplements for eight weeks decreased serum homocysteine to a normal range in patients with venous thrombosis [90].The vitamin intervention for stroke prevention (VISP) randomized controlled trial study demonstrated no significant effect of folate, hydroxocobalamin, and pyridoxine supplementation in decreasing incidence of coronary events or cardiovascular death [91]. The Cochrane systematic review reported no evidence to prevent cardiovascular events by using B vitamins [92].
Carotenoids are a large group of lipid soluble, colorful substances (yellow, orange, and red) such as α-carotene, β-carotene, β-cryptoxanthine, luteine and lycopene which occur extensively in fruit and vegetables [9].
They scavenge free radicals and prevent LDL peroxidation. β-Carotene can decrease plasma cholesterol levels by inhibiting HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase). In addition, carotenoids are capable of increasing macrophage LDL receptor activity and reducing circulating LDL, inflammation, oxidative stress, and endothelial dysfunction [93][94]. A clinical study suggested an inverse relationship between the intake of β-carotene or retinol and risk of cardiovascular disease [95]. The US Preventative Task Force does not suggest β-carotene for the prevention of cardiovascular disease [96]. The Cochrane review on antioxidant consumption indicated that β-carotene and vitamin A significantly increase all-cause mortality [97].
Polyphenols are the most abundant natural antioxidants possessing variable phenolic structures. They are found in fruit (especially apples), grains, vegetables, cereals, olive oil, dry legumes, chocolate and beverages, such as tea, coffee and wine [98]. These compounds are divided into several classes: Flavonoids, phenolic acids (e.g., caffeic acid and gallic acid), stilbenes (e.g., resveratrol), and lignans (e.g., secoisolariciresinol) [9]. Flavonoids, which are a major class of polyphenols, are subclassified as flavonols (e.g., quercetin), flavones (e.g., apigenin, luteolin), flavanones (naringenin, hesperetin), flavan-3-ols (catechins and their oligomers: Proanthocyanidins), isoflavones (e.g., genstein), and anthocyanins (e.g., delphinidin, cyanidin) [9][98].
The mechanistic effects of polyphenols involve: Suppressing ROS formation, scavenging ROS (both radical and non-radical oxygen), increasing the expression level of eNOS and the generation of NO or reducing NO oxidation by enhancing the intracellular free calcium concentration and by activating estrogen receptors in endothelial cells (ECs), blocking the action of xanthine oxidase and protein kinase C to prevent the production of the superoxide radical, and the protection of vascular endothelial cells and NO from oxidation. They also decrease redox-sensitive gene activation, preventing the expression of two major pro-angiogenic factors (vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2)) in smooth muscle cells, increase the production of major vasodilatory factors (NO, endothelium-derived hyperpolarizing factor (EDHF) as well as prostacyclin), inhibit angiogenesis (cell migration and proliferation of blood vessels), and also reduce platelet aggregation and hypertension [99][100][101][102][103][104].
Another important polyphenol that has received much attention is resveratrol (3, 5, 40-trihydroxystilbene), a stilbene polyphenol, which occurs in grapes, red wine and Polygonum cuspidatum. Resveratrol has established antioxidant properties which include the inhibition of lipid oxidation, the regulation of vasodilator and vasoconstrictor production, the inhibition of platelet aggregation, and the inhibition of the transcription factors NF-κB (Nuclear Factor kappaB) and AP-1 (Activator Protein 1) through an interaction with upstream signaling pathways and/or by decreasing pro-inflammatory mediators (TNF-α, IL) [98][105]. Clinical studies such as the Zupthen Elderly study showed a significant inverse association between flavonoid intake and coronary heart disease after 5 years of consumption [106][107]. In addition, the Rotterdam study revealed a significant inverse relationship between total flavonoid intake from the diet with myocardial infarction incidence [108]. The consumption of cocoa or chocolate is inversely associated with carotid atherosclerosis [109].
Probucol (2,6-di-tert-butyl-4-({2-[3,5-di-tert-butyl-4-hydroxyphenyl)sulfanyl) propan-2-yl}
sulfanyl)phenol) is a phenolic lipid-soluble antioxidant [7]. Its activities related to any antiatherosclerotic effectiveness consist of anti-inflammatory activity, the augmentation of endothelial function and repair, lessening oxidant production in vessel walls, attenuating atherosclerosis through the inhibition of LDL oxidation by blocking the production of oxidizing intermediates, inducing heme oxygenase-1 (HO-1) in arterial cells, inhibiting vasomotor dysfunction and fatty streak formation [81], reducing restenosis [7][110][111], inhibiting smooth muscle cell proliferation and adhesion molecule expression on endothelial cells, and promoting endothelium-dependent vasomotion [7].
Another important lipophilic and synthetic antioxidant is BO-653n (2, 3-dihydro-5-hydroxy-2,
2-dipentyl-4,6-di-tert-butylbenzofuran) (an analog of α-tocopherol) which inhibits the formation of atherosclerotic lesions [26], reduces α- tocopheroxyl radical and inhibits LDL oxidation in the intimal area [23][110][112][113].
Atherosclerosis is a major cause of morbidity and mortality in the developed world. Due to the factor that ROS and the generation of oxidized LDL are leading contributors to the progression of atherosclerosis, dietary supplements and antioxidants with low adverse effects may well represent a good therapeutic strategy to prevent the progression of the disease. Natural and synthetic antioxidants facilitate atherosclerosis treatment through a variety of mechanisms, including the inhibition of LDL oxidation, the reduction of generated reactive oxygen species, the inhibition of cytokine secretion, the prevention of atherosclerotic plaque formation and platelet aggregation, the prevention of mononuclear cell infiltration, the improvement of endothelial dysfunction and vasodilation, the promotion of NO bioavailability, the modulation of the expression of adhesion molecules such as VCAM-1 and ICAM-1 on endothelial cells, and the suppression of foam cell formation. It is not clear which of these different mechanisms of antioxidants action is more effective, but it seems that the use of multiple antioxidants is more effective target for antioxidant therapy.