
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcelo Catarino | + 1751 word(s) | 1751 | 2021-07-02 09:38:14 | | | |
| 2 | Vicky Zhou | Meta information modification | 1751 | 2021-07-21 10:32:31 | | | | |
| 3 | Marcelo Catarino | -4 word(s) | 1747 | 2023-10-13 15:57:54 | | |
Video Upload Options
Phlorotannins represent an important group of phenolic compounds, exclusively occurring in brown algae that can form simple structures of 126 Da to very large and complex polymers. Although the biosynthetic pathway of these compounds is still not consensual, it is known that they are formed via C–C and/or C–O–C oxidative coupling of several monomeric units of phloroglucinol, which in turn is known to be biosynthesized through the acetate–malonate pathway. According to the type of linkage formed between these units and the number of hydroxyl groups, phlorotannins can be classified in four sub-classes, namely phlorethols and fuhalols (ether linkages), fucols (aryl-aryl linkages), fucophlorethols (aryl-aryl and ether linkages), and eckols and carmalols (dibenzodoxine linkage).
1. Introduction
Phlorotannins are essentially phenolic compounds biosynthesized exclusively by brown macroalgae that serve vital roles in their physiology including structural functions and cell wall rigidification, response to biotic stress (working as herbivore deterrents, algicidals and bactericidals), and abiotic stress (as photoprotective agents against ultraviolet radiation or as heavy metals chelators). They also participate in the regulation of cell functions and survivability, acting as antioxidants, and in the algal embryogenesis contributing for the zygote’s cell walls formation and avoiding multiple fertilizations by inhibiting spermatozoid movement [1][2].
Unbound phlorotannins are known to accumulate in physodes, i.e., specialized membrane-bound vesicles of the cell cytoplasm, reaching up to concentrations of 25% of seaweed’s DW [3]. The content and profile of phlorotannins in seaweeds are, however, highly susceptible to variability. For instance, some authors demonstrated that seaweeds growing at shallower depths tend to accumulate higher phlorotannin contents than those growing at lower depths [4][5][6], while others have reported that the peak of phlorotannins accumulation in seaweeds usually occur during the spring/summer season [6][7][8]. In both scenarios the higher concentrations of phlorotannins seems to occur in seaweeds under higher solar exposure, which is in agreement with other works that reported an increase of phlorotannins accumulation in seaweeds after UV irradiation or in seaweed tissues with more light exposure [9][10][11]. Water salinity also seems to influence phlorotannins accumulation since higher concentrations of these compounds were reported on seaweeds growing on habitats with higher salinities rather than lower salinities waters [12]. Several other factors such as genetic differences, environmental conditions, nutrient availability, geographical origin, harvesting/post-harvesting/extraction conditions and others have great influence on phlorotannins accumulation, and therefore their concentration in seaweeds usually fluctuates greatly even within the same species.
During the recent years these compounds have gathered great interest due to their vast array of bioactive properties and benefits to human health showing promising effects against oxidative stress, photodamage, cancer, allergy, diabetes, obesity, inflammation, viral, fungal and microbial infections, and several others [13].
2. Biosynthesis and Chemistry of Phlorotannins
Although the exact biosynthetic pathway by which phloroglucinol gives origin to phlorotannins is not yet fully understood, it is known that this compound is biosynthesized by the acetate-malonate pathway, also known as polyketide pathway, in a process involving polyketide synthases-type enzyme complexes (Figure 1). It starts with conversion of an acetyl-CoA molecule into malonyl-CoA by the addition of a carbon dioxide molecule, changing the acetyl group into a highly reactive methylene that will allow the polymerization process to occur with low energy expenditure. The polymerization gives rise to a polyketic chain that transforms into a hexacyclic ring (triketide) via intramolecular cyclization with the concomitant loss of a water molecule. Because the triketide structure is not stable, the molecule tautomerizes into its aromatic form, which is thermodynamically more stable, giving phloroglucinol (1,3,5-trihydroxybenzene) [14]. Phloroglucinol then undergoes through oxidative coupling reactions with other phloroglucinol moieties forming polymeric structures that may range from simple molecules of 126 Da (1 phloroglucinol unit) to very large and complex polymers [15].
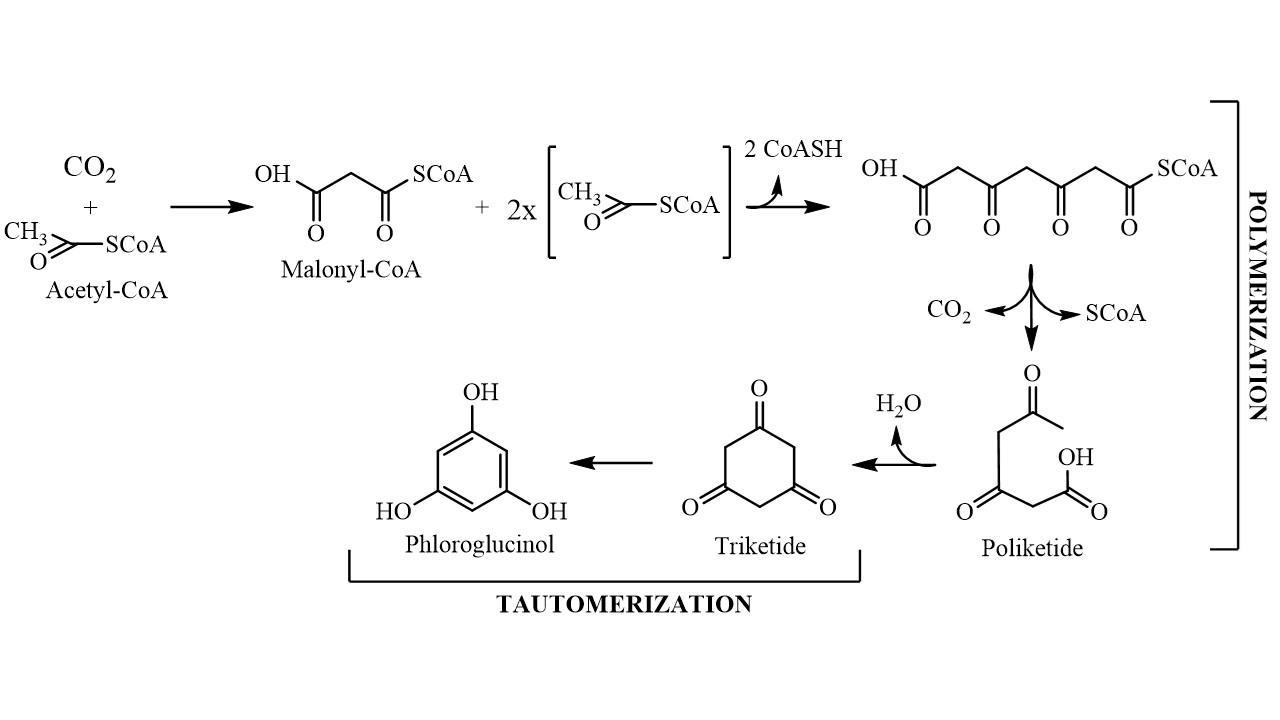
Figure 1. Schematic representation of the phloroglucinol biosynthetic pathway
According to the nature of the structural linkages between phloroglucinol units, they can be characterized into different subclasses namely phlorethols (containing only ether linkages), fucols (containing only C-C linkages), fucophlorethols (containing both ether and C-C linkages) and eckols (containing dibenzodioxin linkages). Within the subclass of phlorethols there are also the fuhalols which are ether-linked phlorotannins that possess at least one additional hydroxyl group. Likewise, eckols with additional hydroxyl groups are known as carmalols (Figure 2) [16].
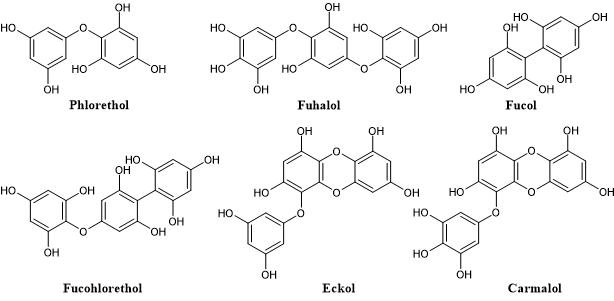
Figure 2. Representation of the different classes of phlorotannins
3. Extraction, Purification and Quantification of Phlorotannins
The extraction of phlorotannins is a demanding task due to their chemical structure complexity, susceptibility to oxidation and interaction with other matrix components. Many factors, such as solvent composition, solvent polarity, time of extraction, temperature, solvent-solid ratio and particle size, may significantly influence the solid–liquid extraction (SLE) of phenolic compounds [17]. The protocols most commonly used for the extraction of phlorotannins are typically based on aqueous mixtures of acetone, ethanol or methanol, usually performed in acidic conditions, under nitrogen atmosphere or with solvents containing antioxidants such as K2S2O5 or ascorbic acid to prevent oxidative degradation [18]. Alternative methods such as pressurized liquid extraction (PLE), microwave-assisted extraction (MAE) or ultrasound-assisted extraction (UAE) have also been tested, although their applicability to routine isolation of phlorotannins is still not very common, due to the necessity of complex apparatus with limited capacities and/or high costs.
The purification of these compounds is usually carried out using solvent partitioning, solid-phase extraction (SPE) or column chromatography for polarity-based separation [19][20][21], or molecular size discrimination through dialysis or ultrafiltration steps [22][23][24]. Other methods have also been reported including cellulose adsorption [25], thin-layer chromatography [26] or even the combination of both polarity- and size-based approaches [23][27].
Monitorization of the total phlorotannins content in the extracts is usually carried out with the Folin-Ciocalteu method. However, even though phlorotannins are the major phenolic compounds present in brown seaweeds, several authors have reported the presence of other non-phlorotannin phenolic compounds in brown seaweeds. Therefore, this is not the most suitable approach since these minor phenolics, together with other unspecific reactions inherent to this colorimetric assay (e.g. with reducing sugars or ascorbic acid) may result in the overestimation of the phlorotannins levels. The most appropriate methodology for the specific quantification of phlorotannins is the dimethoxybenzaldehyde (DMBA) assay. In this reaction, the 2,4-dimethoxybenzaldehyde reacts specifically with the OH groups positioned at the 1,3- and 1,3,5-substituted phenols, thus being much less sensitive to the interference of other compounds. It can, however, form chromophores with some other non-polar compounds, although this issue can be easily surpassed with a defatting step prior to the DMBA assay [28].
4. Bioactive properties of phlorotannins
4.1. Role in Oxidative Stress
4.2. Role in Inflammation
Phlorotannins have also been closely related to the prevention of numerous inflammatory events, having the capacity to interfere with pro-inflammatory cytokines, regulate the expression and/or activity of important enzymes and even intervene in the transcriptional regulation. Many researchers have demonstrated that phlorotannins are capable of inhibiting the expression of cellular adhesion molecules, like ICAM-1 and VCAM-1 and key cytokines such as TNF-α, IL-1β and IL-6, as well as the release of NO● and PG2 and the expression of the enzymes responsible for their synthesis, i.e., iNOS and COX-2 [58][59][60][61][62]. Moreover, phlorotannins have been repeatedly shown both in vitro and in vivo to interfere with the transcriptional activity of NF-κB [63][58][64][65].
Alternatively, phlorotannins are also capable of activating the intracellular anti-inflammatory mechanisms, for example, via stimulation of the expression of IL-10, an important cytokine that is responsible for the supression of cytokines secretion [66].
4.3. Role in Cancer
5. Concluding Remarks
References
- Koki Nagayama; Toshiyuki Shibata; Ken Fujimoto; Tuneo Honjo; Takashi Nakamura; Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 2003, 218, 601-611, 10.1016/s0044-8486(02)00255-7.
- Valeriya Lemesheva; Elena Tarakhovskaya; Physiological functions of phlorotannins. Biological Communications 2018, 63, 70-76, 10.21638/spbu03.2018.108.
- Riitta Koivikko; Jyrki Loponen; Tuija Honkanen; Veijo Jormalainen; Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. Journal of Chemical Ecology 2005, 31, 195-212, 10.1007/s10886-005-0984-2.
- Victoria A Fairhead; Charles D Amsler; James B McClintock; Bill J Baker; Variation in phlorotannin content within two species of brown macroalgae (Desmarestia anceps and D. menziesii) from the Western Antarctic Peninsula. Polar Biology 2005, 28, 680-686, 10.1007/s00300-005-0735-4.
- Klervi Le Lann; Claire Ferret; Elise VanMee; Charlène Spagnol; Marie Lhuillery; Claude Payri; Valérie Stiger‐Pouvreau; Total phenolic, size-fractionated phenolics and fucoxanthin content of tropical Sargassaceae (Fucales, Phaeophyceae) from the South Pacific Ocean: Spatial and specific variability. Phycological Research 2012, 60, 37-50, 10.1111/j.1440-1835.2011.00634.x.
- Solène Connan; Fabienne Goulard; Valérie Stiger; Eric Deslandes; Erwan Ar Gall; Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Botanica Marina 2004, 47, 410-416, 10.1515/bot.2004.057.
- Sabine Parys; Stefan Kehraus; Romain Pete; Frithjof C. Küpper; Karl-Werner Glombitza; Gabriele M. König; Seasonal variation of polyphenolics inAscophyllum nodosum(Phaeophyceae). European Journal of Phycology 2009, 44, 331-338, 10.1080/09670260802578542.
- Mitsunobu Kamiya; Takeshi Nishio; Asami Yokoyama; Kousuke Yatsuya; Tomokazu Nishigaki; Shinya Yoshikawa; Kaori Ohki; Seasonal variation of phlorotannin in sargassacean species from the coast of the Sea of Japan. Phycological Research 2010, 58, 53-61, 10.1111/j.1440-1835.2009.00558.x.
- Michael Y. Roleda; Christian Wiencke; Ulrike H. Lüder; Impact of ultraviolet radiation on cell structure, UV-absorbing compounds, photosynthesis, DNA damage, and germination in zoospores of Arctic Saccorhiza dermatodea. Journal of Experimental Botany 2006, 57, 3847-3856, 10.1093/jxb/erl154.
- Edgardo Cruces; Pirjo Huovinen; Iván Gómez; Interactive effects of UV radiation and enhanced temperature on photosynthesis, phlorotannin induction and antioxidant activities of two sub-Antarctic brown algae. Marine Biology 2012, 160, 1-13, 10.1007/s00227-012-2049-8.
- Ivan Gómez; Pirjo Huovinen; Induction of Phlorotannins During UV Exposure Mitigates Inhibition of Photosynthesis and DNA Damage in the Kelp Lessonia nigrescens. Photochemistry and Photobiology 2010, 86, 1056-1063, 10.1111/j.1751-1097.2010.00786.x.
- Solène Connan; Dagmar B. Stengel; Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquatic Toxicology 2011, 104, 1-13, 10.1016/j.aquatox.2011.03.016.
- Marcelo D. Catarino; Artur M. S. Silva; Susana M. Cardoso; Fucaceae: A Source of Bioactive Phlorotannins. International Journal of Molecular Sciences 2017, 18, 1327, 10.3390/ijms18061327.
- Jihane Achkar; Mo Xian; Huimin Zhao; J. W. Frost; Biosynthesis of Phloroglucinol. Journal of the American Chemical Society 2005, 127, 5332-5333, 10.1021/ja042340g.
- Isuru Wijesekara; Na Young Yoon; Se-Kwon Kim; Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. BioFactors 2010, 36, 408-414, 10.1002/biof.114.
- Inder Pal Singh; Sandip B. Bharate; Phloroglucinol compounds of natural origin. Natural Product Reports 2006, 23, 558-591, 10.1039/b600518g.
- Asaithambi Shakthi Deve; Thiyagarajan Sathish Kumar; Kuppamuthu Kumaresan; Vinohar Stephen Rapheal; Extraction process optimization of polyphenols from Indian Citrus sinensis – as novel antiglycative agents in the management of diabetes mellitus. Journal of Diabetes & Metabolic Disorders 2014, 13, 11-11, 10.1186/2251-6581-13-11.
- José Hipólito Isaza Martínez; Harlen Gerardo Torres Castañeda; Preparation and Chromatographic Analysis of Phlorotannins. Journal of Chromatographic Science 2013, 51, 825-838, 10.1093/chromsci/bmt045.
- Lidia Montero; Miguel Herrero; Elena Ibáñez; Alejandro Cifuentes; Separation and characterization of phlorotannins from brown algaeCystoseira abies-marinaby comprehensive two-dimensional liquid chromatography. ELECTROPHORESIS 2014, 35, 1644-1651, 10.1002/elps.201400133.
- Yajing Li; Xiaoting Fu; Delin Duan; Xiaoyong Liu; Jiachao Xu; Xin Gao; Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Marine Drugs 2017, 15, 49, 10.3390/md15020049.
- Haiyan Liu; Liwei Gu; Phlorotannins from Brown Algae (Fucus vesiculosus) Inhibited the Formation of Advanced Glycation Endproducts by Scavenging Reactive Carbonyls. Journal of Agricultural and Food Chemistry 2012, 60, 1326-1334, 10.1021/jf204112f.
- Tao Wang; Rosa Jonsdottir; Haiyan Liu; Liwei Gu; Hordur G. Kristinsson; Sivakumar Raghavan; Gudrun Olafsdottir; Antioxidant Capacities of Phlorotannins Extracted from the Brown Algae Fucus vesiculosus. Journal of Agricultural and Food Chemistry 2011, 60, 5874-5883, 10.1021/jf3003653.
- Florian Breton; Stéphane Cérantola; Erwan Ar Gall; Distribution and radical scavenging activity of phenols in Ascophyllum nodosum (Phaeophyceae). Journal of Experimental Marine Biology and Ecology 2011, 399, 167-172, 10.1016/j.jembe.2011.01.002.
- Natalie Heffernan; Nigel P. Brunton; Richard J. Fitzgerald; Thomas J. Smyth; Profiling of the Molecular Weight and Structural Isomer Abundance of Macroalgae-Derived Phlorotannins. Marine Drugs 2015, 13, 509-528, 10.3390/md13010509.
- Mariana Barbosa; Graciliana Lopes; Federico Ferreres; Paula B. Andrade; David M. Pereira; Ángel Gil-Izquierdo; Patrícia Valentão; Phlorotannin extracts from Fucales: Marine polyphenols as bioregulators engaged in inflammation-related mediators and enzymes. Algal Research 2017, 28, 1-8, 10.1016/j.algal.2017.09.009.
- Toshiyuki Shibata; Ken Fujimoto; Kohki Nagayama; Kuniko Yamaguchi; Takashi Nakamura; Inhibitory activity of brown algal phlorotannins against hyaluronidase. International Journal of Food Science & Technology 2002, 37, 703-709, 10.1046/j.1365-2621.2002.00603.x.
- Michelle S. Tierney; Thomas Smyth; Dilip K. Rai; Anna Soler-Vila; Anna Croft; Nigel Brunton; Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chemistry 2013, 139, 753-761, 10.1016/j.foodchem.2013.01.019.
- J. Lewis Stern; Ann E. Hagerman; Peter D. Steinberg; Frank C. Winter; James A. Estes; A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. Journal of Chemical Ecology 1996, 22, 1273-1293, 10.1007/bf02266965.
- Ahn, G.-N.; Kim, K.-N.; Cha, S.-H.; Song, C.-B.; Lee, J.; Heo, M.-S.; Yeo, I.-K.; Lee, N.-H.; Jee, Y.-H.; Kim, J.-S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79.
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998.
- He, Y.-Q.; Zhang, W.-T.; Shi, C.-H.; Wang, F.-M.; Tian, X.-J.; Ma, L.-L. Phloroglucinol protects the urinary bladder via inhibition of oxidative stress and inflammation in a rat model of cyclophosphamide-induced interstitial cystitis. Chin. Med. J. 2015, 128, 956–962.
- Jun, Y.-J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.-H.; Kim, H.-R. Eckol enhances heme oxygenase-1 expression through activation of Nrf2/JNK pathway in HepG2 Cells. Molecules 2014, 19, 15638–15652.
- Kang, M.C.; Wijesinghe, W.A.J.P.; Lee, S.H.; Kang, S.M.; Ko, S.C.; Yang, X.; Kang, N.; Jeon, B.T.; Kim, J.; Lee, D.H.; et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type II diabetes in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298.
- Liu, X.; Yuan, W.; Sharma-shivappa, R.; Zanten, J. Van Antioxidant activity of phlorotannins from brown algae. Int. J. Agric. Biol. Eng. 2017, 10, 184–191.
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883.
- Nakai, M.; Kageyama, N.; Nakahara, K.; Miki, W. Phlorotannins as radical scavengers from the extract of Sargassum ringgoldianum. Mar. Biotechnol. 2006, 8, 409–414.
- Sylvia Colliec-Jouault; Catherine Boisson-Vidal; Jacqueline Jozefonvicz; A low molecular weight fucoidan fraction from the brown seaweed Pelvetia canaliculata. Phytochemistry 1994, 35, 697-700, 10.1016/s0031-9422(00)90590-9.
- Seiichirou Nakayasu; Ryo Soegima; Kenichi Yamaguchi; Tatsuya Oda; Biological Activities of Fucose-Containing Polysaccharide Ascophyllan Isolated from the Brown AlgaAscophyllum nodosum. Bioscience, Biotechnology, and Biochemistry 2009, 73, 961-964, 10.1271/bbb.80845.
- Ryogo Abu; Zedong Jiang; Mikinori Ueno; Shogo Isaka; Satoru Nakazono; Takasi Okimura; Kichul Cho; Kenichi Yamaguchi; Daekyung Kim; Tatsuya Oda; et al. Anti-metastatic effects of the sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on B16 melanoma. Biochemical and Biophysical Research Communications 2015, 458, 727-732, 10.1016/j.bbrc.2015.01.061.
- Junzeng Zhang; Christa Tiller; Jingkai Shen; Can Wang; Gabrielle S. Girouard; Dorothy Dennis; Colin J. Barrow; Mingsan Miao; H. Stephen Ewart; Antidiabetic properties of polysaccharide- and polyphenolic-enriched fractions from the brown seaweedAscophyllum nodosumThis article is one of a selection of papers published in this special issue (part 2 of 2) on the Safety and Efficacy of Natural Health Products.. Canadian Journal of Physiology and Pharmacology 2007, 85, 1116-1123, 10.1139/y07-105.
- Emmanouil Apostolidis; Chong M Lee; In Vitro Potential of Ascophyllum nodosum Phenolic Antioxidant-Mediated α-Glucosidase and α-Amylase Inhibition. Journal of Food Science 2010, 75, H97-H102, 10.1111/j.1750-3841.2010.01544.x.
- Sinéad Lordan; Thomas J. Smyth; Anna Soler-Vila; Catherine Stanton; R. Paul Ross; The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chemistry 2013, 141, 2170-2176, 10.1016/j.foodchem.2013.04.123.
- R Boulho; J Le Roux; C Le Quemener; As Burlot; G Audo; N. Bourgougnon; G. Bedoux; Preparative separation of marine bioactive compounds by centrifugal partition chromatography. Planta Medica 2016, 81, S1-S381, 10.1055/s-0036-1596623.
- Seung-Hong Lee; You-Jin Jeon; Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129-136, 10.1016/j.fitote.2013.02.013.
- Yong-Xin Li; Isuru Wijesekara; Se-Kwon Kim; Phlorotannins as bioactive agents from brown algae. Process Biochemistry 2011, 46, 2219-2224, 10.1016/j.procbio.2011.09.015.
- Riitta Koivikko; Jyrki Loponen; Tuija Honkanen; Veijo Jormalainen; Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implication on their ecological functions. Journal of Chemical Ecology 2005, 31, 195-212, 10.1007/s10886-005-0984-2.
- Sofia A. Wikström; Henrik Pavia; Chemical settlement inhibition versus post-settlement mortality as an explanation for differential fouling of two congeneric seaweeds. Oecologia 2003, 138, 223-230, 10.1007/s00442-003-1427-9.
- Balch, P.A.; Bell, S.. Prescription for Herbal Healing; Penguin Group Inc.: London, UK, 2012; pp. 656.
- Nancy M. Targett; Thomas M. Arnold; Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. Journal of Phycology 1998, 34, 195-205, 10.1046/j.1529-8817.1998.340195.x.
- H Pavia; G Cervin; A Lindgren; P Åberg; Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Marine Ecology Progress Series 1997, 157, 139-146, 10.3354/meps157139.
- S. D. Hankins; H. P. Hockey; The effect of a liquid seaweed extract from Ascophyllum nodosum (Fucales, Phaeophyta) on the two-spotted red spider mite Tetranychus urticae. Hydrobiologia 1990, 204-205, 555-559, 10.1007/bf00040286.
- Susan Løvstad Holdt; Stefan Kraan; Bioactive compounds in seaweed: functional food applications and legislation. Environmental Biology of Fishes 2011, 23, 543-597, 10.1007/s10811-010-9632-5.
- Shulan Li; Juan Liu; Mengya Zhang; Yuan Chen; Tianxing Zhu; Jun Wang; Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice. Marine Drugs 2018, 16, 300, 10.3390/md16090300.
- Min-Cheol Kang; Sung-Myung Kang; Ginnae Ahn; Kil-Nam Kim; Nalae Kang; Kalpa W. Samarakoon; Myung-Cheol Oh; Jung-Suck Lee; You-Jin Jeon; Protective effect of a marine polyphenol, dieckol against carbon tetrachloride-induced acute liver damage in mouse. Environmental Toxicology and Pharmacology 2013, 35, 517-523, 10.1016/j.etap.2013.02.013.
- Min-Cheol Kang; W.A.J.P. Wijesinghe; Seung-Hong Lee; Sung-Myung Kang; Seok-Chun Ko; Xiudong Yang; Nalae Kang; Byong-Tae Jeon; Jaell Kim; Dae-Ho Lee; et al.You-Jin Jeon Dieckol isolated from brown seaweed Ecklonia cava attenuates type ІІ diabetes in db/db mouse model. Food and Chemical Toxicology 2013, 53, 294-298, 10.1016/j.fct.2012.12.012.
- Eun-Jeong Yang; Sangzin Ahn; Junghwa Ryu; Moon-Seok Choi; Shinkyu Choi; Young Hae Chong; Jin-Won Hyun; Moon-Jeong Chang; Hye-Sun Kim; Phloroglucinol Attenuates the Cognitive Deficits of the 5XFAD Mouse Model of Alzheimer’s Disease. PLOS ONE 2015, 10, e0135686, 10.1371/journal.pone.0135686.
- Yoshie-Stark, Y Hsieh, YP Suzuki, Takeshi; Distribution of flavonoids and related compounds from seaweeds in Japan. Tokyo University of Fisheries 2003, 89, 1-6.
- Moon-Moo Kim; Se-Kwon Kim; Effect of phloroglucinol on oxidative stress and inflammation. Food and Chemical Toxicology 2010, 48, 2925-2933, 10.1016/j.fct.2010.07.029.
- Sang-Hoon Lee; Sung-Hwan Eom; Na-Young Yoon; Moon-Moo Kim; Yong-Xin Li; Sang Keun Ha; Se-Kwon Kim; Fucofuroeckol-A fromEisenia bicyclisInhibits Inflammation in Lipopolysaccharide-Induced Mouse Macrophages via Downregulation of the MAPK/NF-κB Signaling Pathway. Journal of Chemistry 2016, 2016, 1-9, 10.1155/2016/6509212.
- Won-Kyo Jung; Soo-Jin Heo; You-Jin Jeon; Chang-Min Lee; Yeong-Min Park; Hee-Guk Byun; Yung Hyun Choi; Sae-Gwang Park; Il-Whan Choi; Inhibitory Effects and Molecular Mechanism of Dieckol Isolated from Marine Brown Alga on COX-2 and iNOS in Microglial Cells. Journal of Agricultural and Food Chemistry 2009, 57, 4439-4446, 10.1021/jf9003913.
- Young-Mi Kang; Sung-Hwan Eom; Young-Mog Kim; Protective effect of phlorotannins from Eisenia bicyclis against lipopolysaccharide-stimulated inflammation in HepG2 cells. Environmental Toxicology and Pharmacology 2013, 35, 395-401, 10.1016/j.etap.2013.01.009.
- A-Reum Kim; Tai-Sun Shin; Min-Sup Lee; Ji-Young Park; Kyoung-Eun Park; Na-Young Yoon; Jong-Soon Kim; Jae-Sue Choi; Byeong-Churl Jang; Dae-Seok Byun; et al.Nam-Kyu ParkHyeung-Rak Kim Isolation and Identification of Phlorotannins from Ecklonia stolonifera with Antioxidant and Anti-inflammatory Properties. Journal of Agricultural and Food Chemistry 2009, 57, 3483-3489, 10.1021/jf900820x.
- A-Reum Kim; Bonggi Lee; Eun-Ji Joung; Wi-Gyeong Gwon; Tadanobu Utsuki; Nam-Gil Kim; Hyeung-Rak Kim; 6,6′-Bieckol suppresses inflammatory responses by down-regulating nuclear factor-κB activation via Akt, JNK, and p38 MAPK in LPS-stimulated microglial cells. Immunopharmacology and Immunotoxicology 2016, 38, 244-252, 10.3109/08923973.2016.1173060.
- A.-Reum Kim; Min-Sup Lee; Tai-Sun Shin; Hong Hua; Byeong-Churl Jang; Jae-Sue Choi; Dae-Seok Byun; Tadanobu Utsuki; Donald Ingram; Hyeung-Rak Kim; et al. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK. Toxicology in Vitro 2011, 25, 1789-1795, 10.1016/j.tiv.2011.09.012.
- Taddesse Yayeh; Eun Ju Im; Tae-Hyung Kwon; Seong-Soo Roh; Suk Kim; Ji Hye Kim; Seung-Bok Hong; Jae Youl Cho; Nyun-Ho Park; Man Hee Rhee; et al. Hemeoxygenase 1 partly mediates the anti-inflammatory effect of dieckol in lipopolysaccharide stimulated murine macrophages. International Immunopharmacology 2014, 22, 51-58, 10.1016/j.intimp.2014.06.009.
- Paola A. Tenorio-Rodríguez; Hugo Esquivel-Solis; Jesús I. Murillo-Álvarez; Felipe Ascencio; Ángel I. Campa-Córdova; Carlos Angulo; Biosprospecting potential of kelp (Laminariales, Phaeophyceae) from Baja California Peninsula: phenolic content, antioxidant properties, anti-inflammatory, and cell viability. Environmental Biology of Fishes 2019, 31, 3115-3129, 10.1007/s10811-019-01781-1.
- Meng-Ya Zhang; Jie Guo; Xian-Min Hu; Shu-Qi Zhao; Shu-Lan Li; Jun Wang; An in vivo anti-tumor effect of eckol from marine brown algae by improving the immune response. Food & Function 2019, 10, 4361-4371, 10.1039/c9fo00865a.
- Jin-Soo Yoon; Anandam Kasin Yadunandam; Soon-Jin Kim; Hee-Chul Woo; Hyeung-Rak Kim; Gun-Do Kim; Dieckol, isolated from Ecklonia stolonifera, induces apoptosis in human hepatocellular carcinoma Hep3B cells. Journal of Natural Medicines 2012, 67, 519-527, 10.1007/s11418-012-0709-0.
- Ji-Hye Ahn; Yeong-In Yang; Kyung-Tae Lee; Jung-Hye Choi; Dieckol, isolated from the edible brown algae Ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. Journal of Cancer Research and Clinical Oncology 2014, 141, 255-268, 10.1007/s00432-014-1819-8.
- Chang-Suk Kong; Jung-Ae Kim; Na-Young Yoon; Se-Kwon Kim; Induction of apoptosis by phloroglucinol derivative from Ecklonia Cava in MCF-7 human breast cancer cells. Food and Chemical Toxicology 2009, 47, 1653-1658, 10.1016/j.fct.2009.04.013.
- Seung Hyun Jung; In Seung Jang; You-Jin Jeon; Young-Mog Kim; Sun Joo Park; Dieckol Suppresses CoCl 2 -induced Angiogenesis in Endothelial Cells. Fisheries and aquatic sciences 2014, 17, 305-311, 10.5657/fas.2014.0305.
- Velayutham Sadeeshkumar; Arul Duraikannu; Samuthrapandian Ravichandran; Paulrasu Kodisundaram; Wilson Sylvester Fredrick; Rajagopal Gobalakrishnan; Modulatory efficacy of dieckol on xenobiotic-metabolizing enzymes, cell proliferation, apoptosis, invasion and angiogenesis during NDEA-induced rat hepatocarcinogenesis. Molecular and Cellular Biochemistry 2017, 433, 195-204, 10.1007/s11010-017-3027-8.
- Moon-Moo Kim; Quang Van Ta; Eresha Mendis; Niranjan Rajapakse; Won-Kyo Jung; Hee-Guk Byun; You-Jin Jeon; Se-Kwon Kim; Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sciences 2006, 79, 1436-1443, 10.1016/j.lfs.2006.04.022.
- Eun-Kyung Kim; Yujiao Tang; Yon-Suk Kim; Jin-Woo Hwang; Eun-Ju Choi; Ji-Hyeok Lee; Seung-Hong Lee; You-Jin Jeon; Pyo-Jam Park; First Evidence that Ecklonia cava-Derived Dieckol Attenuates MCF-7 Human Breast Carcinoma Cell Migration. Marine Drugs 2015, 13, 1785-1797, 10.3390/md13041785.
- Chun‐Hong Wang; Xiao‐Feng Li; Li‐Fang Jin; Yan Zhao; Geng‐Jun Zhu; Wei‐Zhang Shen; Dieckol inhibits non-small-cell lung cancer cell proliferation and migration by regulating the PI3K/AKT signaling pathway.. Journal of Biochemical and Molecular Toxicology 2019, 33, e22346, 10.1002/jbt.22346.
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012.
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, anti-inflammatory and antiproliferative effects of aqueous extracts of three mediterranean brown seaweeds of the Genus Cystoseira. Iran. J. Pharm. Res. 2014, 13, 207–220.
- Eun-Kyung Kim; Yujiao Tang; Yon-Suk Kim; Jin-Woo Hwang; Eun-Ju Choi; Ji-Hyeok Lee; Seung-Hong Lee; You-Jin Jeon; Pyo-Jam Park; First Evidence that Ecklonia cava-Derived Dieckol Attenuates MCF-7 Human Breast Carcinoma Cell Migration. Marine Drugs 2015, 13, 1785-1797, 10.3390/md13041785.




