Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angeline Van Dongen | + 1254 word(s) | 1254 | 2021-05-26 09:32:54 | | | |
| 2 | Vicky Zhou | Meta information modification | 1254 | 2021-06-29 03:10:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Van Dongen, A. Froth Treatment Tailings. Encyclopedia. Available online: https://encyclopedia.pub/entry/11401 (accessed on 08 February 2026).
Van Dongen A. Froth Treatment Tailings. Encyclopedia. Available at: https://encyclopedia.pub/entry/11401. Accessed February 08, 2026.
Van Dongen, Angeline. "Froth Treatment Tailings" Encyclopedia, https://encyclopedia.pub/entry/11401 (accessed February 08, 2026).
Van Dongen, A. (2021, June 28). Froth Treatment Tailings. In Encyclopedia. https://encyclopedia.pub/entry/11401
Van Dongen, Angeline. "Froth Treatment Tailings." Encyclopedia. Web. 28 June, 2021.
Copy Citation
Froth treatment tailings (FTT) are a specific type of tailings waste stream from the bitumen froth treatment process that contains bioavailable diluent: either naphtha or paraffins. Tailings ponds that receive FTT are associated with the highest levels of biogenic gas production, as diverse microbial communities biodegrade the residual diluent.
froth treatment tailings
diluent
microbial communities
GHGs
characterization
biodegradation
1. Introduction
FTT are a waste stream of the oil sands extraction process. In order to extract useable hydrocarbons from oil sands deposits, the oil sands ore must first undergo treatment to separate the bitumen in oil sands from the water and sand. During primary extraction, oil sands are mixed with water, hydrocarbon diluent, and air to form a slurry [1]. Bitumen is then separated from the slurry by flotation, resulting in a bitumen-rich froth that also contains fine solids and emulsified water droplets [1][2]. During the subsequent froth treatment process, diluent is added to reduce the density and viscosity of the bitumen, thereby enhancing separation of the hydrocarbons from the solids and water [2]. These waste products become FTT. This extraction process is conceptually illustrated in Figure 1.
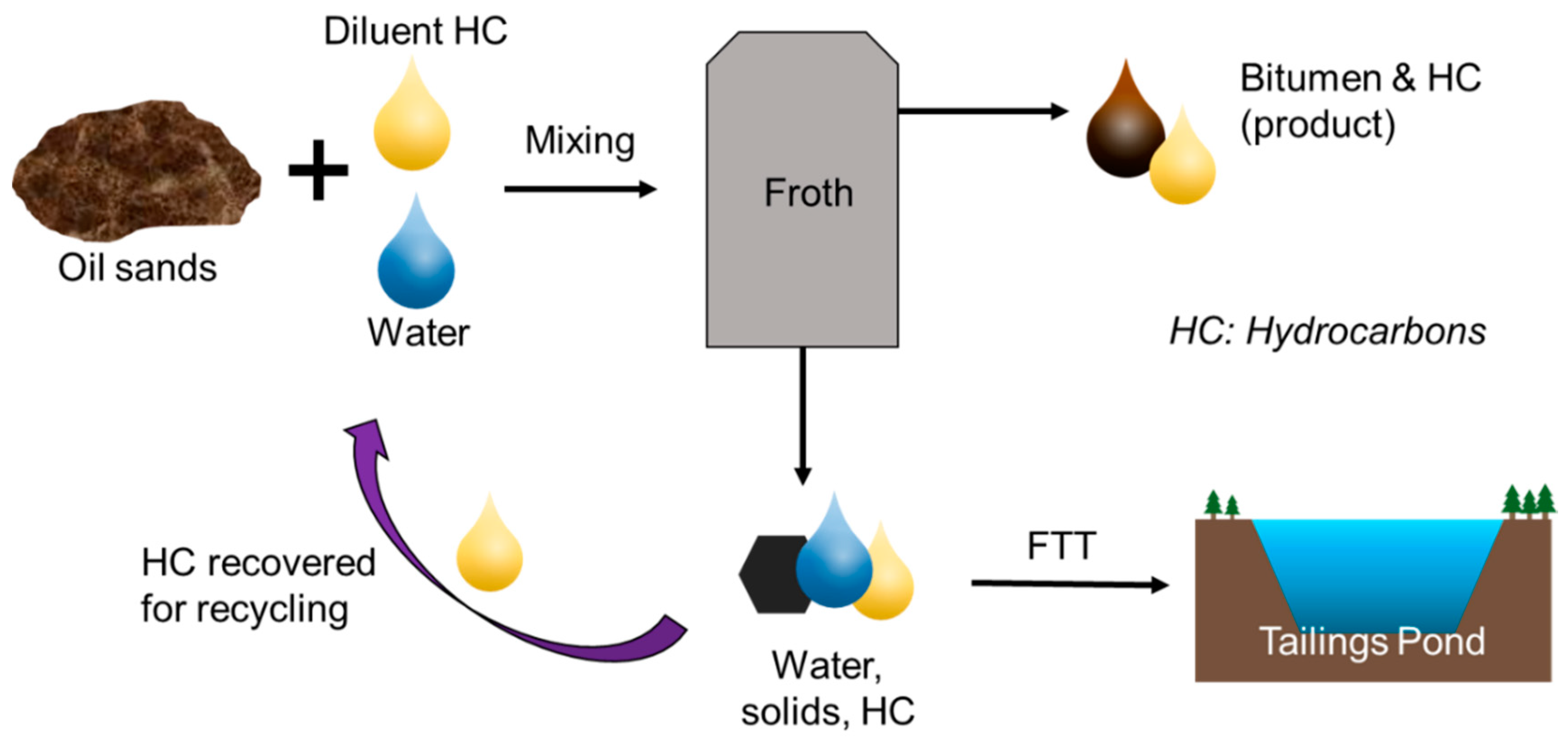 Figure 1. Primary separation process of bitumen from oil sands.
Figure 1. Primary separation process of bitumen from oil sands.Two types of organic diluents are mainly used for froth treatment: naphtha and paraffinic solvents [3]. Naphtha refers to a mixture of C5–C12 aliphatic hydrocarbons and benzene, toluene, ethylbenzene and xylene (BTEX) compounds [4][5]. Paraffinic diluent is composed mainly of C5 and C6 alkanes [6]. The type of diluent chosen in the froth treatment process is based on the desired bitumen product quality [7]. Naphtha-based froth treatment has been predominantly used in the oil sands for over 30 years and typically requires a naphtha/bitumen ratio of 0.6–0.75 w/w [2][8]. The relatively newer paraffinic-based froth treatment process produces a cleaner bitumen product; however, this is achieved at a lower bitumen recovery rate and requires a higher diluent/bitumen ratio above 1.5 w/w [2]. After froth treatment, the diluent is recovered in solvent recovery units for reuse, but a fraction ends up in the FTT waste stream [9][3]. The exact chemical composition of FTT depends on the ore, extraction process, refining process, and additives used [10]. FTT are typically composed of 76.5 wt% water, 17 wt% mineral solid particles, 4.5 wt% bitumen, and up to 2 wt% diluent [2]. The mineral solid particles in FTT are primarily silicates with varying amounts of oxides, carbonates, sulphides, and sulphates [11]. FTT can also contain BTEX, polycyclic aromatic hydrocarbons (PAHs), hazardous metals, and naturally occurring radioactive minerals [12][11][13].
FTT are deposited in tailings ponds in the same ways as other types of extraction tailings: either subaqueously or subaerially onto the pond surface [6][8][11]. Solids and larger asphaltene components of the tailings settle quickly, forming a layer of sand and sediment on the beaches and bottom of tailings ponds, surrounding the fine clay particles, which are extremely slow to settle and are referred to as fluid fine tailings (FFT) [8][14]. Tailings are typically stored in ponds for years to allow for further dewatering and consolidation [15]. Once the fine tailings solid content reaches 30–40% w/w, these tailings are referred to as mature fine tailings (MFT) [14].
2. Types of Froth Treatment Tailings
2.1. Naphtha FTT
Naphtha is a complex mixture of low molecular weight n-, iso-, and cycloalkanes and monoaromatics (BTEX) [16][17]. Naphtha is produced during the bitumen upgrading process and is recovered for use as diluent [18]. Naphtha composition varies between operators; some use heavy naphtha containing mostly C9–C16 aliphatics, while others use light sour naphtha with mainly C5–C8, or naphtha containing primarily C6–C10 hydrocarbons [12][19]. An analysis of heavy naphtha found it contained 18 wt% n-alkanes, 31 wt% iso-alkanes, 27 wt% cycloalkanes and 15 wt% BTEX [9]. The residual naphtha concentration in tailings ponds from different operators ranges between 0.2 and 0.5 wt% [20][9][19][21]. After the bitumen extraction and naphtha recovery processes, the composition of residual naphtha released in FTT generally contains higher amounts of less volatile, heavier hydrocarbons [9].
2.2. Paraffinic FTT
Compared to naphtha, paraffinic diluent is relatively simple and is composed primarily of C5 and C6 n- and iso-alkanes [22][17]. Analysis of paraffinic diluent found that it contained 24 wt% n-pentane, 11 wt% n-hexane, and 49 wt% iso-alkanes (2-methylbutane, 2-methylpentane, and 3-methylpentane) [23].
Paraffinic FTT composition differs from naphthenic FTT in a few other ways. Paraffinic solvents precipitate the asphaltene component of bitumen, which act as flocculants, further removing suspended solids and water droplets to achieve a cleaner bitumen product containing less moisture and solids [2][3]. The precipitated asphaltene aggregates are collected in the paraffinic FTT [2]. Additionally, paraffinic FTT contain trisodium citrate, which is added during the extraction process instead of NaOH, and polyacrylamide, which is added to thicken tailings before deposition in tailings ponds [6]. Trisodium citrate is an easily fermentable methanogenic substrate that may also contribute to tailings pond emissions [6][24].
3. Final Remarks
In summary, FTT are a type of tailings generated by the bitumen froth treatment process that contain either naphthenic or paraffinic diluent. The biodegradation of diluent in FTT is the primary contributor to biogenic emissions from tailings ponds. Tailings ponds that receive FTT streams generally have higher GHG emissions, VOC emissions, and higher potential to generate RSCs depending on microbial community, tailings composition, and pond physical properties. Although tailings ponds harbor dynamic microbial populations that are highly diverse due to tailings characteristics, some bacterial and archaeal taxa, notably Peptococcaceae and Methanosaetaceae, have been found consistently in various studies regardless of diluent type and incubation time. Microbial inhabitants of tailings can degrade large fractions of residual diluent. Simple hydrocarbons are preferably metabolized first, followed by relatively complex hydrocarbons and finally more complex and recalcitrant hydrocarbons. Diluent degradation takes place in a series of steps involving a syntrophic partnership between fermentative bacteria and methanogenic archaea through hydrogenotrophic and/or acetoclastic pathways. The biodegradation of diluent and its constituents by microbial communities in tailings has been well studied in laboratory experiments, however, several areas require further research in order to better understand the complex chemical and microbial process contributing to emissions from tailings ponds receiving FTT.
In most of the laboratory studies, tailings samples were spiked with pure diluent, whereas the actual residual diluent concentration and composition in FTT is variable and less well studied. The analysis of diluent remaining in tailings samples, particularly FTT, is a critical step in determining the effect of hydrocarbons on the microbial activity in tailings ponds and associated GHG emissions. While a somewhat patchy body of literature exists regarding the extraction and analysis of hydrocarbons, a comprehensive study of solvents, extraction conditions, and GC parameters is missing. When approaching analysis of FTT, we must rely on techniques that were developed for different tailings types. A systematic study of extraction parameters for FTT would yield the most accurate data and ensure that data is not being biased by using ill-suited conditions.
Predicting GHG, RSC, and VOC emissions from tailings ponds is difficult due to the heterogeneity and complexity within tailings ponds. Further research is needed to confirm predicted VOC losses associated with different diluent types. For assessing GHG emissions from distinct diluent hydrocarbons, stoichiometric models have been developed, but they are not well supported by field data. Until now, most studies have relied on the pyrosequencing method of DNA sequencing, however, advanced sequencing techniques with more in-depth coverage is vital to understand the vast complexity of tailings microbes. Additional research is required to capture the spatial and temporal patterns of FTT-associated microbial activity to better predict GHGs and guide tailings pond management decisions.
References
- Romanova, U.G.; Valinasab, M.; Stasiuk, E.N.; Yarranton, H.W.; Schramm, L.L.; Shelfantook, W.E. The Effect of Oil Sands Bitumen Extraction Conditions on Froth Treatment Performance. J. Can. Pet. Technol. 2006, 45, 10.
- Rao, F.; Liu, Q. Froth Treatment in Athabasca Oil Sands Bitumen Recovery Process: A Review. Energy Fuels 2013, 27, 7199–7207.
- Gray, M.R. Upgrading Oilsands Bitumen and Heavy Oil, 1st ed.; The University of Alberta Press: Edmonton, AB, Canada, 2015.
- Siddique, T.; Kuznetsov, P.; Kuznetsova, A.; Arkell, N.; Young, R.; Li, C.; Guigard, S.; Underwood, E.; Foght, J.M. Microbially-Accelerated Consolidation of Oil Sands Tailings. Pathway I: Changes in Porewater Chemistry. Front. Microbiol. 2014, 5.
- Prestvik, R.; Kjell, M.; Grande, K.; Holmen, A. Compositional Analysis of Naphtha and Reformate. In Catalytic Naphtha Reforming; CRC Press: Boca Raton, FL, USA, 2004.
- Foght, J.M.; Gieg, L.M.; Siddique, T. The Microbiology of Oil Sands Tailings: Past, Present, Future. FEMS Microbiol. Ecol. 2017, 93.
- Shelfantook, W.E. A Perspective on the Selection of Froth Treatment Processes. Can. J. Chem. Eng. 2008, 82, 704–709.
- Xu, Y.; Dabros, T.; Kan, J. Investigation on Alternative Disposal Methods for Froth Treatment Tailings-Part 1, Disposal without Asphaltene Recovery. Can. J. Chem. Eng. 2013, 91, 1349–1357.
- Siddique, T.; Fedorak, P.M.; MacKinnon, M.D.; Foght, J.M. Metabolism of BTEX and Naphtha Compounds to Methane in Oil Sands Tailings. Environ. Sci. Technol. 2007, 41, 2350–2356.
- Small, C.C.; Cho, S.; Hashisho, Z.; Ulrich, A.C. Emissions from Oil Sands Tailings Ponds: Review of Tailings Pond Parameters and Emission Estimates. J. Pet. Sci. Eng. 2015, 127, 490–501.
- Lindsay, M.B.J.; Vessey, C.J.; Robertson, J.M. Mineralogy and Geochemistry of Oil Sands Froth Treatment Tailings: Implications for Acid Generation and Metal(Loid) Release. Appl. Geochem. 2019, 102, 186–196.
- Burkus, Z.; Wheler, J.; Pletcher, S. GHG Emissions from Oil Sands Tailings Ponds: Overview and Modelling Based on Fermentable Substrates. Part. I: Review of the Tailings Ponds Facts and Practices; Environment and Sustainable Resource Development: Edmonton, AB, Canada, 2014.
- Moran, K.; Nelson, S.; Oxenford, J. New Oil Sands Technology to Meet the Challenges of Climate Change and Tailings Management; World Heavy Oil Congress: Calgary, AB, Canada, 2016.
- Voordouw, G. Interaction of Oil Sands Tailings Particles with Polymers and Microbial Cells: First Steps toward Reclamation to Soil. Biopolymers 2013, 99, 257–262.
- Dompierre, K.A.; Lindsay, M.B.J.; Cruz-Hernández, P.; Halferdahl, G.M. Initial Geochemical Characteristics of Fluid Fine Tailings in an Oil Sands End Pit Lake. Sci. Total Environ. 2016, 556, 196–206.
- Abu Laban, N.; Dao, A.; Semple, K.; Foght, J. Biodegradation of C7 and C8 Iso-Alkanes under Methanogenic Conditions: Methanogenic Iso-Alkane Biodegradation. Environ. Microbiol. 2015, 17, 4898–4915.
- Mohamad Shahimin, M.F.; Foght, J.M.; Siddique, T. Preferential Methanogenic Biodegradation of Short-Chain n-Alkanes by Microbial Communities from Two Different Oil Sands Tailings Ponds. Sci. Total Environ. 2016, 553, 250–257.
- Charry-Sanchez, J.; Betancourt-Torcat, A.; Ricardez-Sandoval, L. An Optimization Energy Model for the Upgrading Processes of Canadian Unconventional Oil. Energy 2014, 68, 629–643.
- Mohamad Shahimin, M.F.; Siddique, T. Sequential Biodegradation of Complex Naphtha Hydrocarbons under Methanogenic Conditions in Two Different Oil Sands Tailings. Environ. Pollut. 2017, 221, 398–406.
- Siddique, T.; Fedorak, P.M.; Foght, J.M. Biodegradation of Short-Chain n-Alkanes in Oil Sands Tailings under Methanogenic Conditions. Environ. Sci. Technol. 2006, 40, 5459–5464.
- Siddique, T.; Penner, T.; Semple, K.; Foght, J.M. Anaerobic Biodegradation of Longer-Chain n-Alkanes Coupled to Methane Production in Oil Sands Tailings. Environ. Sci. Technol. 2011, 45, 5892–5899.
- Siddique, T.; Kuznetsova, A. Linking Hydrocarbon Biodegradation to Greenhouse Gas Emissions from Oil Sands Tailings and Its Impact on Tailings Management. Can. J. Soil Sci. 2020, 100, 537–545.
- Mohamad Shahimin, M.F.; Siddique, T. Methanogenic Biodegradation of Paraffinic Solvent Hydrocarbons in Two Different Oil Sands Tailings. Sci. Total Environ. 2017, 583, 115–122.
- Li, C. Methanogenesis in Oil Sands Tailings: An Analysis of the Microbial Community Involved and Its Effects on Tailings Densification. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2010.
More
Information
Subjects:
Mineralogy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
29 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




