
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kenneth K. Wu | + 3725 word(s) | 3725 | 2021-05-07 04:02:19 | | | |
| 2 | Vivi Li | -1726 word(s) | 1999 | 2021-05-10 09:36:16 | | |
Video Upload Options
Cytoguardin was identified in the conditioned medium of fibroblasts as a tryptophan metabolite, 5-methoxytryptophan (5-MTP). It is synthesized via two enzymatic steps: tryptophan hydroxylase (TPH) and hydroxyindole O-methyltransferase (HIOMT).
1. Introduction
Cytoguardin is a cyclooxygenase-2 (COX-2) suppressing factor which was discovered in the conditioned medium (CM) of proliferating fibroblasts (P-Fb) [1][2]. It was noted that COX-2 expression in P-Fb was reduced when compared to that in quiescent fibroblasts (Q-Fb) due to release of a suppressing factor into the CM [2]. The analysis of a purified fraction of the CM by NMR revealed small molecule weight compounds with an indole moiety [2]. As the indole-containing molecules in CM were considered to defend against cellular and tissue damage by COX-2 overexpression, they were named cytoguardins [2]. Cytoguardin activity was not detected in the CM of several cancer cell lines including A549 lung cancer cells, HCT29 colorectal cancer cells, HepB2 hepatocellular cancer cells, and MCF7 breast cancer cells. This finding offers an opportunity to use comparative metabolomics to resolve the chemical identity of cytoguardin. A distinct peak on mass spectrometry is present in the extract of P-Fb CM, but not in that of A549 cancer cells [2]. Investigations with biochemical and molecular genetic approaches identified this peak as 5-methoxytryptophan (5-MTP) [3]. Chemo-synthetic L-5MTP inhibits COX-2 expression in a manner resembling endogenous cytoguardin. Thus, 5-MTP is a key chemical component, if not the only component, of cytoguardin.
5-MTP is produced not only in fibroblasts but also in vascular endothelial cells (ECs), smooth muscle cells (SMCs), and bronchial and renal epithelial cells [4]. Its synthesis is catalyzed by tryptophan hydroxylase (TPH) which converts L-tryptophan to 5-hydroxytryptophan (5-HTP) and hydroxyindole O-methyltransferase (HIOMT) which converts 5-HTP to 5-MTP [3]. 5-MTP is secreted via the Golgi vesicular transport system [4]. 5-MTP acts in a paracrine and autocrine manner to control cancer cell COX-2 expression, cancer cell migration, and cancer growth and metastasis [3]. In addition, it defends against macrophage activation and the release of cytokines [4], and protects the vascular endothelium from damage and leaking [5][6]. Its multitude of actions are mediated by common mechanisms including the inhibition of the p38 MAPK signaling pathway and NF-κB and p300 transcriptional activation [4][7]. Its anti-inflammatory and vasoprotective actions were summarized in a recent review [8]. This review will focus on the anti-tumor actions of 5-MTP. It will summarize the defects of HIOMT expression and deficiency of 5-MTP production in cancer cells, and the metabolic switch and restoration of 5-MTP production and control of cancer cell COX-2 expression and cancer growth and metastasis by HIOMT stable transfection. The mechanisms by which 5-MTP inhibits cancer cell COX-2 expression and reduces cancer growth and metastasis are discussed.
2. Deficient Cancer Cell 5-MTP Production Due to Defects of HIOMT Expression
The quantitative analysis of 5-MTP levels by liquid chromatography-mass spectrometry (LC-MS) or enzyme-immunoassay detects very low 5-MTP level in cancer cell CM. As cancer cells express abundant TPH-1 but are unable to convert 5-HTP to 5-MTP, reduced 5-MTP levels are attributed to the defective expression of HIOMT [3]. HIOMT was identified in pineal tissues as the final enzymatic step in melatonin biosynthesis [9]. It catalyzes the conversion of N-acetylserotonin (N-acetyl 5-hydroxytryptamine) to melatonin (N-acetyl 5-methoxytryptamine), and hence it is commonly known as N-acetylserotonin methyltransferase (ASMT). Human ASMT was reported to be encoded by a single gene [10]. However, three mRNA isoforms are detected in pineal cells: isoform 373, which codes for a 373-amino acid (aa) protein, is considered to be the full-length isoform while isoforms 345 and 298 are spliced products in which exon 6 and exon 6 & 7 are spliced, respectively [11]. There is suggestive evidence that isoform 345 is a wild-type ASMT (HIOMT). First, exon 6 in isoform 373 contains repeat LINE-1 sequences and is considered as an insertion from transposons [11]. It is thus possible that isoform 373 may be an insertion mutant. The HIOMT373 isoform is not expressed in other mammalian cells. In fact, bovine and macaque pineal cells express a single mRNA which aligns with the human HIOMT345 isoform. Mouse and rat cells also express a single HIOMT mRNA which aligns with human HIOMT345, but the sequence is more divergent than that of bovine or macaque. Sequence comparison suggests that the 345 isoform is a conserved isoform for melatonin biosynthesis. This is supported by structure-function analysis. Bovine and macaque pineal cells express only ASMT345 which shares with human ASMT345 a high degree of sequence identity. Human 345 isoform was reported to be catalytically active in melatonin synthesis, while isoform 298 and 373 are inactive [12]. ASMT345 is the predominant isoform detected in human pineal and retinal cells. Although ASMT373 and 298 are also detected, albeit at a low level, their functional roles are unclear.
Human fibroblasts and ECs express a single HIOMT isoform which is identical to pineal cell ASMT298 [13]. Cancer cells express a minute quantity of HIOMT298 [13]. The analysis of HIOMT proteins in human cancer tissue arrays reveals a low level of HIOMT in a majority of colorectal (CRC), pancreatic, and breast cancer tissues [13]. It is unclear why cancer cells are defective in HIOMT expression. This could be due to aberrant promoter function or mRNA instability. It is clear, however, that HIOMT298 is active in catalyzing 5-MTP synthesis as stable transfection of A549 cells and with HIOMT298 restores HIOMT expression and 5-MTP production in A549 cancer cells [13]. A deficiency of 5-MTP in cancer cells due to HIOMT expression defects contributes to cancer cell migratory and proliferative activities.
3. HIOMT-Transfected Cancer Cells Undergo a Metabolic Switch from Serotonin to 5-MTP Production
Quantitative analysis of 5-HTP metabolites in A549 CM reveals abundant serotonin but scarce 5-MTP and undetectable melatonin [13]. Serotonin was reported to promote growth of hepatocellular, breast, and prostate cancer [14][15]. Serotonin promotes cancer growth by binding to selective receptors, notably 5-HT receptor 2B, via which it activates ERK [16]. Serum serotonin was reported to be a poor prognostic biomarker of breast cancer [17]. Serotonin stimulates prostate cancer cell growth via 5-HT receptor 1A, 1B, 2B, and 4 [18][19][20]. It has been reported that serotonin receptor antagonists inhibit prostate cancer growth [18][19][20]. Serotonin appears to play a dual role in CRC development. It was reported to be crucial for CRC cancer growth by inducing angiogenesis [21]. On the other hand, it protects mouse colonic crypt from DNA damage and CRC tumorigenesis [22].
HIOMT298 transfection of A549 cells results in a drastic change in the metabolic profile of the CM. The 5-MTP level becomes highly elevated while serotonin is suppressed and melatonin remains undetectable [13]. Metabolite changes in the CM are correlated with alteration in the enzyme expression: abundant HIOMT298 and diminished expression of aromatic L-amino acid decarboxylase (AADC) (Figure 1). AADC catalyzes the conversion of 5-HTP to 5-hydroxytryptamine (5-HT, serotonin). It is also known as L-DOPA decarboxylase (DDC) as it converts L-DOPA to dopamine [23][24][25]. AADC is expressed in the neurons and cells of peripheral tissues. The neuronal and non-neuronal expression of AADC is driven by distinct promoters due to alternative splicing [26]. Two AADC transcripts are identified which code for AADC 480 and AADC 442 proteins [27]. AADC480 is catalytically active in serotonin synthesis. Cancer cells of neuroendocrine origin such as small cell lung cancer cells (SCLC) express a high level of neuronal type AADC [28]. A549 cells which are non-neuroendocrine cells express a lower level of AADC than SCLC cells; non-neuronal type AADC mRNA is predominant [28]. AADC mRNA levels are reduced by HIOMT298 transfection [13]. It is unclear how HIOMT298 transfection elicits a suppressing effect of AADC expression. It was reported that AADC expression driven by non-neuronal promoters is regulated by hepatocyte nuclear factor 1 (HNF-1) [29]. It remains to be determined whether HIOMT298/5-MTP suppresses AADC expression via this regulatory transcriptional mechanism. The metabolic switch exerts a great influence on cancer cell malignant behavior. It enhances the anti-tumor effect of 5-MTP.
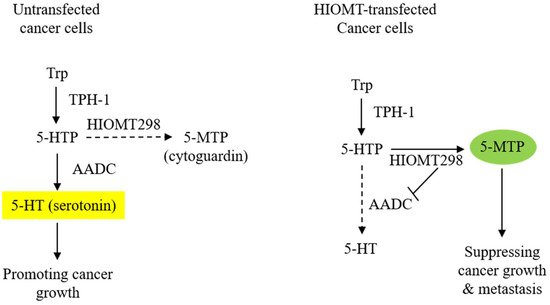
Figure 1. Transfection of A549 cells with HIOMT298 switches 5-hydroxytryptophan (5-HTP) catabolism. The left panel shows AADC/serotonin as the major pathway in untransfected A549 cells and the right panel shows the switch of 5-HTP metabolites from serotonin to 5-MTP synthesis due to the suppression of AADC in HIOMT298-overexpressed A549 cells. Dotted lines denote reduced reaction.
4. 5-MTP Inhibits Cancer Growth and Metastasis through the Control of COX-2 Expression
COX-2 is constitutively expressed in many types of human cancers. Constitutive COX-2 expression in cancer cells is driven by complex mechanisms [30]. Several studies have shown that cancer cells exhibit increased β-catenin/T cell factor binding and β-catenin-mediated COX-2 promoter activity [31][32]. The upregulation of pontin52 was implicated as an endogenous driver of β-catenin transactivation [33]. Pontin52 is a nuclear protein which interacts with β-catenin and binds to the TATA box binding protein [34]. Human cancer expresses high levels of pontin52, via which β-catenin mediated COX-2 expression is enhanced. p53 has been implicated in COX-2 expression in cancer cells; p53 upregulates COX-2 expression in cultured colon and breast cancer cells at the transcriptional level [35]. Constitutive COX-2 expression in cancer cells could also be due to increased COX-2 mRNA stability in cancer cells as a result of an increased expression of HuR which stabilizes COX-2 mRNA and increases COX-2 mRNA translation [36].
COX-2 expression in cancer cells is augmented by stimulation with phorbol 12 myristate 13 acetate (PMA), pro-inflammatory cytokines, and growth factors. The COX-2-inducing factors enhance COX-2 transcription via the activation of transactivators such as NF-κB, C/EBPβ, AP-1, and CREB-2, accompanied by the increased binding of transcriptional co-activator p300 and p300 HAT activity [37][38][39]. Cancer cell COX-2 expression is further augmented by factors produced by host stromal cells in the tumor microenvironment [40].
4.1. COX-2 Overexpression Drives Cancer Growth and Metastasis
Numerous studies have provided evidence for the important pathogenic role that COX-2 plays in cancer progression. COX-2 overexpression in cancer cells has been shown to confer resistance to apoptosis, promote proliferation, induce angiogenesis, and enhance cellular migration and invasion. Most of the tumor-promoting actions of COX-2 are ascribed to its major metabolite, prostaglandin E2 (PGE2) [41]. PGE2 was reported to promote cancer cell proliferation through the trans-activation of epidermoid growth factor receptor (EGFR) [42]; induce angiogenesis via the HIF-1α/VEGF axis [43]; confer apoptosis resistance via Bcl2, p53 and c-Myc [44][45][46]; and enhance cancer cell migration and invasion via pro-inflammatory mediators [47]. The inhibition of COX-2 activity in cancer cells with selective COX-2 inhibitors results in reduced tumor growth in animal models and human clinical trials [48][49]. The genetic silencing of COX-2 in breast cancer cells was reported to block cancer cell migration and invasion, reducing cancer metastasis in a mouse model [50]. Taken together, these reported data indicate that COX-2 expression is causally correlated with cancer growth and metastasis.
Cancer aggressiveness is augmented by COX-2/PGE2-mediated immune suppression. PGE2 induces regulatory T cell (Treg) generation through the expression of indole 2,3-dioxygenase (IDO) or tryptophan 2, 3-dioxygenase (TDO) in cancer cells [51][52]. IDO and TDO catalyze kynurenine (Kyn) generation from L-tryptophan. Kyn not only induces Treg but also promotes cancer growth and invasion via the aryl hydrocarbon receptor [40][53].
4.2. 5-MTP Inhibits Cancer Cell COX-2 Expression and Cancer Progression and Metastasis
5-MTP inhibits COX-2 expression in A549 cancer cells in a concentration-dependent manner [3]. The effects of 5-MTP on cancer cell COX-2 expression and cancer growth have been evaluated in a murine xenograft tumor model. Intraperitoneal administration of 5-MTP reduces tumor growth and at seven weeks after the subcutaneous implantation of A549 cells, tumor volume was reduced by 50% when compared to the vehicle control [3]. Metastatic lung nodules are reduced by 5-MTP. The analysis of COX-2 in subcutaneous cancer cells reveals the suppression of COX-2 expression in 5-MTP-treated mice. 5-MTP is effective in controlling cancer growth and metastasis through the reduction of COX-2 expression.
4.3. 5-MTP Inhibits COX-2 Transcription by Blocking p300 HAT Activation and Transactivator Binding
It is unclear how 5-MTP inhibits cancer cell COX-2 expression. However, it is known that 5-MTP inhibits PMA-induced COX-2 expression in fibroblasts by blocking the binding of NF-κB, C/EBPβ, AP-1, and CREB to the COX-2 promoter [7]. Furthermore, 5-MTP inhibits p300 HAT activity whereby it amplifies its inhibition of COX-2 transcription. It was reported that cancer cells exhibit aberrant autonomous activation of NF-IL6 and CRE [30]. It is possible that 5-MTP inhibits cancer cell COX-2 expression by controlling the binding of C/EBPβ and CREB to the NF-IL6 and CRE cis-acting elements on the promoter of COX-2 and related genes.
References
- Gilroy, D.W.; Saunders, M.A.; Sansores-Garcia, L.; Matijevic-Aleksic, N.; Wu, K.K. Cell cycle-dependent expression of cyclooxygenase-2 in human fibroblasts. FASEB J. 2001, 15, 288–290.
- Deng, W.G.; Saunders, M.; Gilroy, D.; He, X.Z.; Yeh, H.; Zhu, Y.; Shtivelband, M.I.; Ruan, K.H.; Wu, K.K. Purification and characterization of a cyclooxygenase-2 and angiogenesis suppressing factor produced by human fibroblasts. FASEB J. 2002, 16, 1286–1288.
- Cheng, H.H.; Kuo, C.C.; Yan, J.L.; Chen, H.L.; Lin, W.C.; Wang, K.H.; Tsai, K.K.; Guvén, H.; Flaberg, E.; Szekely, L.; et al. Control of cyclooxygenase-2 expression and tumorigenesis by endogenous 5-methoxytryptophan. Proc. Natl. Acad. Sci. USA 2012, 109, 13231–13236.
- Wang, Y.F.; Hsu, Y.J.; Wu, H.F.; Lee, G.L.; Yang, Y.S.; Wu, J.Y.; Yet, S.F.; Wu, K.K.; Kuo, C.C. Endothelium-derived 5-methoxytryptophan is a circulating anti-inflammatory molecule that blocks systemic inflammation. Circ. Res. 2016, 119, 222–236.
- Ho, Y.C.; Wu, M.L.; Su, C.H.; Chen, C.H.; Ho, H.H.; Lee, G.L.; Lin, W.S.; Lin, W.Y.; Hsu, Y.J.; Kuo, C.C.; et al. A Novel Protective Function of 5-Methoxytryptophan in Vascular Injury. Sci. Rep. 2016, 6, 25374.
- Chu, L.Y.; Wang, Y.F.; Cheng, H.H.; Kuo, C.C.; Wu, K.K. Endothelium-derived 5-methoxytryptophan protects endothelial barrier function by blocking p38 MAPK activation. PLoS ONE 2016, 11, e0152166.
- Cheng, H.H.; Wang, K.H.; Chu, L.Y.; Chang, T.C.; Kuo, C.C.; Wu, K.K. Quiescent and proliferative fibroblasts exhibit differential p300 HAT activation through control of 5-methoxytryptophan production. PLoS ONE 2014, 9, e88507.
- Wu, K.K.; Kuo, C.C.; Yet, S.F.; Lee, C.M.; Liou, J.Y. 5-methoxytryptophan: An arsenal against vascular injury and inflammation. J. Biomed. Sci. 2020, 27, 79.
- Axelrod, J.; Weissbach, H. Enzymatic O-methylation of N-acetylserotonin to melatonin. Science 1960, 31, 1312.
- Rodriguez, I.R.; Mazuruk, K.; Schoen, T.J.; Chader, G.J. Structural analysis of the human hydroxyindole-O-methyltransferase gene. Presence of two distinct promoters. J. Biol. Chem. 1994, 269, 31969–31977.
- Donohue, S.J.; Roseboom, P.H.; Illnerova, H.; Weller, J.L.; Klein, D.C. Human hydroxyindole-O-methyltransferase: Presence of LINE-1 fragment in a cDNA clone and pineal mRNA. DNA Cell Biol. 1993, 12, 715–727.
- Botros, H.G.; Legrand, P.; Pagan, C.; Bondet, V.; Weber, P.; Ben-Abdallah, M.; Lemière, N.; Huguet, G.; Bellalou, J.; Maronde, E.; et al. Crystal structure and functional mapping of human ASMT, the last enzyme of the melatonin synthesis pathway. J. Pineal Res. 2013, 54, 46–57.
- Chen, H.L.; Yuan, C.Y.; Cheng, H.H.; Chang, T.C.; Huang, S.K.; Kuo, C.C.; Wu, K.K. Restoration of hydroxyindole O-methyltransferase levels in human cancer cells induces a tryptophan-metabolic switch and attenuates cancer progression. J. Biol. Chem. 2018, 293, 11131–11142.
- Soll, C.; Jang, J.H.; Riener, M.O.; Moritz, W.; Wild, P.J.; Graf, R.; Clavien, P.A. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology 2010, 51, 1244–1254.
- Sarrouilhe, D.; Clarhaut, J.; Defamie, N.; Mesnil, M. Serotonin and cancer: What is the link? Curr. Mol. Med. 2015, 15, 62–77.
- Liu, S.; Miao, R.; Zhai, M.; Pang, Q.; Deng, Y.; Liu, S.; Qu, K.; Liu, C.; Zhang, J. Effects and related mechanisms of serotonin on malignant biological behavior of hepatocellular carcinoma via regulation of Yap. Oncotarget 2017, 8, 47412–47424.
- Leoncikas, V.; Wu, H.; Ward, L.T.; Kierzek, A.M.; Plant, N.J. Generation of 2,000 breast cancer metabolic landscapes reveals a poor prognosis group with active serotonin production. Sci. Rep. 2016, 6, 19771.
- Abdul, M.; Anezinis, P.E.; Logothetis, C.J.; Hoosein, N.M. Growth inhibition of human prostatic carcinoma cell lines by serotonin antagonists. Anticancer Res. 1994, 14, 1215–1220.
- Dizeyi, N.; Bjartell, A.; Nilsson, E.; Hansson, J.; Gadaleanu, V.; Cross, N.; Abrahamsson, P.A. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate 2004, 59, 328–336.
- Siddiqui, E.J.; Shabbir, M.; Mikhailidis, D.P.; Thompson, C.S.; Mumtaz, F.H. The role of serotonin (5-hydroxytryptamine1A and 1B) receptors in prostate cancer cell proliferation. J. Urol. 2006, 176, 1648–1653.
- Nocito, A.; Dahm, F.; Jochum, W.; Jang, J.H.; Georgiev, P.; Bader, M.; Graf, R.; Clavien, P.A. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008, 68, 5152–5158.
- Sakita, J.Y.; Bader, M.; Santos, E.S.; Garcia, S.B.; Minto, S.B.; Alenina, N.; Brunaldi, M.O.; Carvalho, M.C.; Vidotto, T.; Gasparotto, B.; et al. Serotonin synthesis protects the mouse colonic crypt from DNA damage and colorectal tumorigenesis. J. Pathol. 2019, 249, 102–113.
- Lovenberg, W.; Weissbach, H.; Udenfriend, S. Aromatic L-amino acid decarboxylase. J. Biol. Chem. 1962, 237, 89–93.
- Tanaka, T.; Horio, Y.; Taketoshi, M.; Imamura, I.; Ando-Yamamoto, M.; Kangawa, K.; Matsuo, H.; Kuroda, M.; Wada, H. Molecular cloning and sequencing of a cDNA of rat dopa decarboxylase: Partial amino acid homologies with other enzymes synthesizing catecholamines. Proc. Natl. Acad. Sci. USA 1989, 86, 8142–8146.
- Sumi-Ichinose, C.; Ichinose, H.; Takahashi, E.; Hori, T.; Nagatsu, T. Molecular cloning of genomic DNA and chromosomal assignment of the gene for human aromatic L-amino acid decarboxylase, the enzyme for catecholamine and serotonin biosynthesis. Biochemistry 1992, 31, 2229–2238.
- Albert, V.R.; Lee, M.R.; Bolden, A.H.; Wurzburger, R.J.; Aguanno, A. Distinct promoters direct neuronal and nonneuronal expression of rat aromatic L-amino acid decarboxylase. Proc. Natl. Acad. Sci. USA 1992, 89, 12053–12057.
- O’Malley, K.L.; Harmon, S.; Moffat, M.; Uhland-Smith, A.; Wong, S. The human aromatic L-amino acid decarboxylase gene can be alternatively spliced to generate unique protein isoforms. J. Neurochem. 1995, 65, 2409–2416.
- Vachtenheim, J.; Novotná, H. Expression of the aromatic L-amino acid decarboxylase mRNA in human tumour cell lines of neuroendocrine and neuroectodermal origin. Eur. J. Cancer 1997, 33, 2411–2417.
- Aguanno, A.; Afar, R.; Albert, V.R. Tissue-specific expression of the nonneuronal promoter of the aromatic L-amino acid decarboxylase gene is regulated by hepatocyte nuclear factor 1. J. Biol. Chem. 1996, 271, 4528–4538.
- Shao, J.; Sheng, H.; Inoue, H.; Morrow, J.D.; DuBois, R.N. Regulation of constitutive cyclooxygenase-2 expression in colon carcinoma cells. J. Biol. Chem. 2000, 275, 33951–33956.
- Araki, Y.; Okamura, S.; Hussain, S.P.; Nagashima, M.; He, P.; Shiseki, M.; Miura, K.; Harris, C.C. Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res. 2003, 63, 728–734.
- Nuñez, F.; Bravo, S.; Cruzat, F.; Montecino, M.; De Ferrari, G.V. Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells. PLoS ONE 2011, 6, e18562.
- Carlson, M.L.; Wilson, E.T.; Prescott, S.M. Regulation of COX-2 transcription in a colon cancer cell line by Pontin52/TIP49a. Mol. Cancer 2003, 2, 42.
- Bauer, A.; Huber, O.; Kemler, R. Pontin52, an interaction partner of beta-catenin, binds to the TATA box binding protein. Proc. Natl. Acad. Sci. USA 1998, 95, 14787–14792.
- Corcoran, C.A.; He, Q.; Huang, Y.; Sheikh, M.S. Cyclooxygenase-2 interacts with p53 and interferes with p53-dependent transcription and apoptosis. Oncogene 2005, 24, 1634–1640.
- Dixon, D.A.; Tolley, N.D.; King, P.H.; Nabors, L.B.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J. Clin. Investig. 2001, 108, 1657–1665.
- Wu, K.K. Inducible cyclooxygenase and nitric oxide synthase. Adv. Pharmacol. 1995, 33, 179–207.
- Deng, W.G.; Zhu, Y.; Wu, K.K. Up-regulation of p300 binding and p50 acetylation in tumor necrosis factor-alpha-induced cyclooxygenase-2 promoter activation. J. Biol. Chem. 2003, 278, 4770–4777.
- Deng, W.G.; Zhu, Y.; Wu, K.K. Role of p300 and PCAF in regulating cyclooxygenase-2 promoter activation by inflammatory mediators. Blood 2004, 103, 2135–2142.
- Chen, J.Y.; Li, C.F.; Kuo, C.C.; Tsai, K.K.; Hou, M.F.; Hung, W.C. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2, 3-dioxygenase promotes breast cancer progression. Breast Cancer Res. 2014, 16, 410.
- Wang, D.; DuBois, R.N. Role of prostanoids in gastrointestinal cancer. J. Clin. Investig. 2018, 128, 2732–2742.
- Dannenberg, A.J.; Lippman, S.M.; Mann, J.R.; Subbaramaiah, K.; DuBois, R.N. Cyclooxygenase-2 and epidermal growth factor receptor: Pharmacologic targets for chemoprevention. J. Clin. Oncol. 2005, 23, 254–266.
- Stasinopoulos, I.; O’Brien, D.R.; Bhujwalla, Z.M. Inflammation, but not hypoxia, mediated HIF-1alpha activation depends on COX-2. Cancer Biol. Ther. 2009, 8, 31–35.
- You, Z.; Saims, D.; Chen, S.; Zhang, Z.; Guttridge, D.C.; Guan, K.L.; MacDougald, O.A.; Brown, A.M.; Evan, G.; Kitajewski, J.; et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J. Cell Biol. 2002, 157, 429–440.
- Sheng, H.; Shao, J.; Morrow, J.D.; Beauchamp, R.D.; DuBois, R.N. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998, 58, 362–366.
- Choi, E.M.; Heo, J.I.; Oh, J.Y.; Kim, Y.M.; Ha, K.S.; Kim, J.I.; Han, J.A. COX-2 regulates p53 activity and inhibits DNA damage-induced apoptosis. Biochem. Biophys. Res. Commun. 2005, 328, 1107–1112.
- Jiang, J.; Dingledine, R. Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J. Pharm. Exp. Ther. 2013, 344, 360–367.
- Koehne, C.H.; Dubois, R.N. COX-2 inhibition and colorectal cancer. Semin. Oncol. 2004, 31, 12–21.
- Krysan, K.; Reckamp, K.L.; Sharma, S.; Dubinett, S.M. The potential and rationale for COX-2 inhibitors in lung cancer. Anticancer Agents Med. Chem. 2006, 6, 209–220.
- Stasinopoulos, I.; O’Brien, D.R.; Wildes, F.; Glunde, K.; Bhujwalla, Z.M. Silencing of cyclooxygenase-2 inhibits metastasis and delays tumor onset of poorly differentiated metastatic breast cancer cells. Mol. Cancer Res. 2007, 5, 435–442.
- Basu, G.D.; Tinder, T.L.; Bradley, J.M.; Tu, T.; Hattrup, C.L.; Pockaj, B.A.; Mukherjee, P. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: Role of IDO. J. Immunol. 2006, 177, 2391–2402.
- Ott, M.; Litzenburger, U.M.; Rauschenbach, K.J.; Bunse, L.; Ochs, K.; Sahm, F.; Pusch, S.; Opitz, C.A.; Blaes, J.; von Deimling, A.; et al. Suppression of TDO-mediated tryptophan catabolism in glioblastoma cells by a steroid-responsive FKBP52-dependent pathway. Glia 2015, 63, 78–90.
- Munn, D.H.; Mellor, A.L. IDO and tolerance to tumors. Trends Mol. Med. 2004, 10, 15–18.




