
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hyun-Jae Shin | + 3166 word(s) | 3166 | 2021-04-27 09:00:52 | | | |
| 2 | Vivi Li | + 10 word(s) | 3176 | 2021-05-06 11:39:17 | | |
Video Upload Options
Quercetin is a well-known plant flavonol and antioxidant; however, there has been some debate regarding the efficacy and safety of native quercetin as a skin-whitening agent via tyrosinase inhibition. Several researchers have synthesized quercetin derivatives as low-toxicity antioxidants and whitening agents. However, no suitable quercetin derivatives have been reported to date. In this study, a novel quercetin derivative was synthesized by the SN2 reaction using quercetin and oleyl bromide. The relationship between the structures and activities of quercetin derivatives as anti-melanogenic agents was assessed using in vitro enzyme kinetics, molecular docking, and quenching studies; cell line experiments; and in vivo zebrafish model studies. Novel 3,7-dioleylquercetin (OQ) exhibited a low cytotoxic concentration level at >100 µg/mL (125 µM), which is five times less toxic than native quercetin.
1. Introduction
Melanin is a naturally occurring pigment that produces eyes, hair, and skin color. An amino acid, tyrosine, is required to support the production of melanin. Decreased melanin production may have a protective effect on the skin. The growing demand for whitening agents in cosmetic products has had a significant influence on anti-melanogenesis research. The inhibition of melanin biosynthesis (hypopigmentation or anti-melanogenesis) in the skin plays a critical role in protecting against various skin problems, such as spots, melasma, and freckles. Excessive production and accumulation of melanin (hyperpigmentation and melanogenesis) may cause serious skin diseases, such as skin cancer [1]. Therefore, many scientists have been trying to find a safe and effective whitening agent for cosmetic and therapeutic applications. To date, many potent tyrosinase inhibitors have been isolated, developed, and used in various skincare products, including ascorbic acid, kojic acid, arbutin, hydroquinone, and quercetin [2][3]. Among them, arbutin has been used as a positive control in numerous whitening studies. It is a hydroquinone glycoside with two isoforms, 4-hydroxypheyl-α-glucopyranoside and 4-hydroxylpheyl-β-glucopyranoside. Numerous studies have shown that arbutin is as effective as hydroquinone but less toxic [4]. The alpha isomer has the greatest inhibitory activity against mammalian tyrosinases [5].
As various natural resources show antioxidant activity, phenolic compounds are frequently used as cosmetic ingredients. In particular, quercetin (3,3′,4′,5,7-pentahydroxyl-flavone), a model polyphenol compound, is well known as a strong antioxidant and anti-whitening agent [3][6][7][8]. Quercetin has been widely used as a cosmetic material because it is present in large quantities in many plants. Quercetin is a potent tyrosinase inhibitor, a melanogenesis inhibitor, in several cell lines, such as mouse B16 melanoma, and an antioxidant and anticancer agent. However, there have been some controversies regarding the anti-melanogenesis effect based on the origin, concentration, and assay methods. In addition to native quercetin, several studies on the activity of plant extracts containing quercetin derivatives have been conducted to reduce the toxicity and inhibit tyrosinase activity. Quercetin 4′-O-β-D-glucopyranoside and its extract showed potent tyrosinase inhibitory activity [9][10]. Chao et al. [11] confirmed that the whitening effect was increased by attaching galactose, rhamnose, and xylose to the 3-OH position of quercetin. In addition, Taira et al. [12] verified the whitening effect and toxicity of quercetin-glucose-rhamnose derivatives. Concerning an artificial quercetin derivative, several unsaturated fatty acid-quercetin derivatives were synthesized; however, they showed no superior toxicity or kinetic constants than native quercetin. To achieve the goal of less toxicity and high inhibition activity of tyrosinase, facile synthesis of novel quercetin derivatives is required.
2. Synthesis and Identification of Novel Compounds
Synthesis was performed by modifying the method of Kato et al. [13]. The synthesis of OQ was undertaken via the SN2 reaction using quercetin and oleyl bromide (Scheme 1). Generally, the reaction occurs simultaneously at positions 3, 7, and 4′ of the OH group of native quercetin. We performed the synthesis by adjusting the equivalent molar ratio to attach the oleyl moiety to the 3-OH and 7-OH positions. After the reaction, a mixture of compounds with several oleyl moieties was obtained. The initial reaction products were isolated and assayed for tyrosinase and antioxidant activity. The final single compound was isolated and purified by open column chromatography, medium-pressure liquid chromatography (MPLC), and high-performance liquid chromatography (HPLC) (Supplementary Figure S1, supplementary could be found in https://www.mdpi.com/1422-0067/22/8/4264), and the structure of the purified compound was confirmed by NMR spectroscopy (Figure S2) [14]. The yield of OQ was 11%.
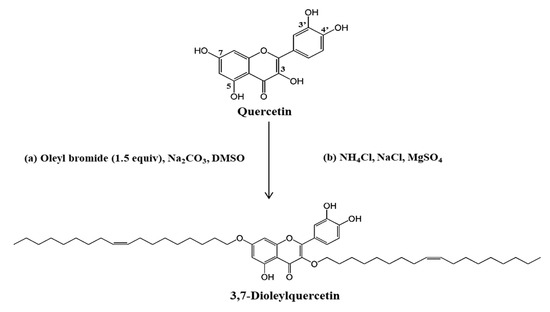
Scheme 1. Synthesis of 3,7-dioleylquercetin (OQ). Oleyl bromide, Na2CO3, DMSO, then rt 30 h. Abbreviation: rt, room temperature.
3. Inhibition Kinetics
The effects of quercetin and OQ on the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) were studied using the method described by Chen and Kubo [15]. The inhibition of tyrosinase by OQ was concentration-dependent, as shown in Figure 1. As the OQ concentration increased, the residual enzyme activity rapidly decreased, but it was not completely suppressed. The inhibition concentration leading to 50% activity loss (IC50) was estimated to be 0.0987 mM, which was the highest among the native quercetin and quercetin derivatives (Table 1). The plot of the residual enzyme activity versus the enzyme concentrations at different OQ concentrations produced a family of straight lines (Figure 1), which passed through the origin, indicating that the inhibition of the enzyme by quercetin was reversible. Increasing the inhibitor (OQ) concentration resulted in a decrease in the slope of the lines. The plot of 1/ν versus 1/[S] produced a series of straight lines, indicating that OQ is a competitive inhibitor. Thus, the kinetic behavior of mushroom tyrosinase during the oxidation of L-DOPA was depicted as a reversible competitive inhibition model, as follows (Scheme 2): Where, E (Emet, Edeoxy, and Eoxy), S, I, and P denote the enzyme (three forms of the enzyme), substrate, inhibitor (quercetin and OQ), and product, respectively. EI and ES are the respective compounds.
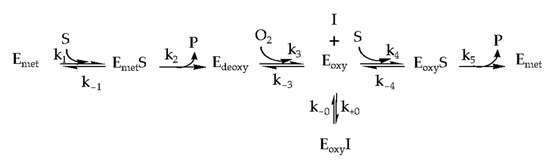
Scheme 2. Enzymatic reaction model with reversible competitive inhibition.
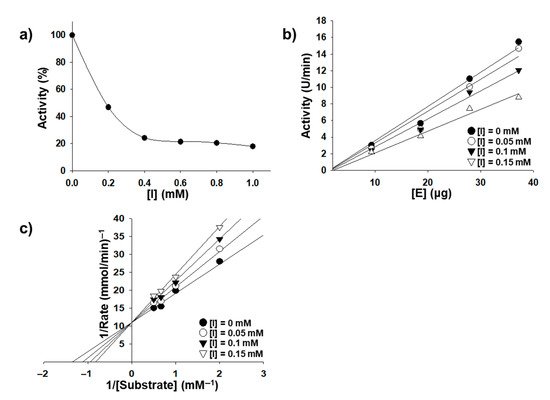
Figure 1. (a) Effect of OQ on the activity of mushroom tyrosinase for the catalysis L-DOPA (enzyme concentration 4.0 μg/mL). (b) Relationship of the catalytic activity of mushroom tyrosinase with the enzyme concentrations at different OQ concentrations. OQ concentrations for curves 1–4 were 0, 0.05, 0.1, and 0.15 mM, respectively. (c) Lineweaver-Burk plots for the inhibition of quercetin on mushroom tyrosinase for the catalysis of DOPA at 30 °C, pH 6.8. Concentrations of quercetin for curves 1–4 were 0, 0.05, 0.1, and 0.15 mM, respectively; the enzyme concentration was 4.0 μg/mL. The inset represents the plot of Kmapp versus the quercetin concentration for determining the inhibition constants KI.
Table 1. Kinetics parameters and microscopic inhibition rate constants of mushroom tyrosinase by native quercetin and quercetin derivatives (3,7-dioleylquercetin and quercetin-7-oleate).
| Native Quercetin a,b | 3,7-dioleylquercetin a | Quercetin-7-oleate c | |
|---|---|---|---|
| IC50 | 0.4714 mM (0.13 mM) | 0.0987 mM | 0.71 mM |
| Km | 0.7524 mM (0.84 mM) | 0.736 mM | 1.11 mM |
| Vm | 75.6 U/min (122 U/min) | 30.2 U/min | 4.98 mM |
| Inhibition | Reversible (reversible) | Reversible | Reversible |
| Inhibition type | Competitive (competitive) | Competitive | Competitive |
| Ki | 0.2459 mM (0.0386mM) | 0.232 mM | 0.43 mM |
| k+0 | 0.0217 mM−1s−1 (0.0216 mM−1s−1) | 0.0298 mM−1s−1 | - |
| 0.0235 mM−1s−1 (0.0219 mM−1s−1) | 0.0272 mM−1s−1 | - | |
| k–0 | 0.49 × 10−2 s−1 (0.832 × 10−3s−1) | 0.43 × 10−2 s−1 | - |
The kinetic parameters of mushroom tyrosinase obtained from the Lineweaver-Burk plot showed that Km was equal to 0.736 mM and Vmax was equal to 30.2 U/min (8.1 μM/min). Further, the Lineweaver-Burk plot was linearly fitted (shown in Figure 1b, implying that OQ had a single inhibitory site or a single class of tyrosinase inhibitory sites [17]. The inhibition constant (Ki) of OQ was calculated to be 0.232 mmol L−1 in the second plot of the apparent Km/Vm or 1/Vm versus OQ concentration (Table 1). As Km and Vmax are known quantities from measurements of the substrate reaction in the absence of the modifier at different substrate concentrations, the microscopic rate constants (k+0 and k − k-0) were easily determined according to the analysis of Tsou’s kinetic model [18] and are summarized in Table 1. Our data showed that the kinetic parameter values, except for Vm, were similar for quercetin and OQ.
4. Effet of OQ on Anti-Melanogenesis-Elated Proteinsin B16F10 Cell Line
Melanogenesis mainly depends on the regulation of melanogenic proteins such as tyrosinase, tyrosinase-related protein 1 (TRP-1), and tyrosinase-related protein 2 (TRP-2). Western blot analysis was performed to determine whether the inhibitory effects of OQ were related to the regulation of melanogenesis-related proteins. In general, microphthalmia-associated transcription factor (MITF) is a well-known master transcription factor of three major pigmentation enzymes: tyrosinase, TPR-1, and TRP-2 [19]. MITF regulates melanocyte differentiation and the transcription of melanogenic enzymes, such as tyrosinase, TRP-1, and TRP-2, to activate or regulate protein expression [19]. As shown in Figure 2a,b, the protein expression levels of tyrosinase, TRP-1, and TRP-2 were increased by the α-MSH treatment, whereas OQ led to a significant decrease in tyrosinase, TRP-1, and TRP-2 in B16F10 cell lines. Moreover, α-MSH-induced (1 µg/mL) MITF expression was decreased in a dose-dependent manner by treatment with OQ at 50–100 µg/mL (62–125 µM) (Figure 3a,b). Therefore, OQ inhibits melanogenesis by downregulating MITF signaling. To elucidate the mechanism underlying the melanogenesis of OQ, B16F10 cells were exposed to OQ (50–100 µg/mL (62–125 µM)) for the indicated incubation time, and the protein extracts were then analyzed by western blotting analysis. As shown in Figure 4, OQ preincubation inhibited α-MSH-induced phosphorylation of protein kinase and cAMP response element-binding protein (PKA/CREB). Therefore, the suppressive mechanism of OQ was related to the inhibition of PKA/CREB signaling.
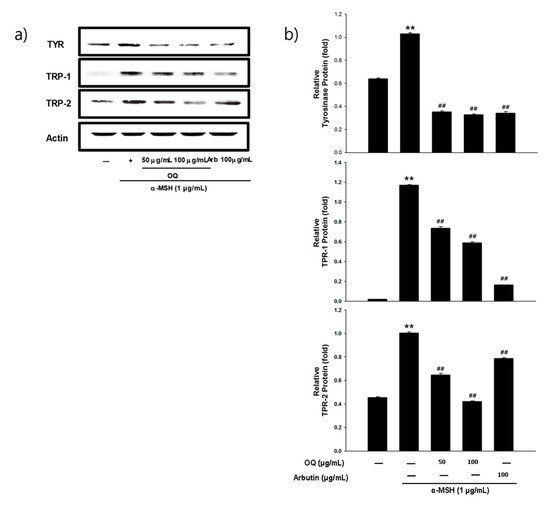
Figure 2. (a) Effect of OQ and arbutin on tyrosinase, TRP-1, and TRP-2 expression in B16F10 melanoma cells. B16F10 cells were treated with the concentrations of the OQ and arbutin prior to α-MSH treatment for 24 h. The loading control was assessed using a β-actin antibody. (b) Quantitative analysis of tyrosinase, TRP-1, and TRP-2 by western blotting. Cell lysates were subjected to western blotting using antibodies against tyrosinase, TRP-1, and TRP-2. Values are significantly different by Duncan’s multiple range test (significant compared to the vehicle-treated control, ** p < 0.01; significant compared to α-MSH, ## p < 0.01, bars indicate S.D.).
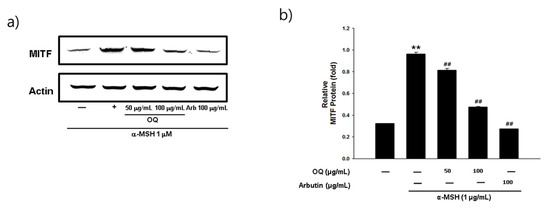
Figure 3. (a) Effect of OQ and arbutin on MITF protein expression in B16F10 cells. B16F10 cells were treated with the indicated concentrations of the OQ and arbutin prior to α-MSH treatment for 4 h. (b) Quantitative analysis of MITF by western blotting. Values are significantly different by Duncan’s multiple range test (significant compared to the vehicle-treated control, ** p < 0.01; significant compared to α-MSH, ## p < 0.01, bars indicate S.D.).
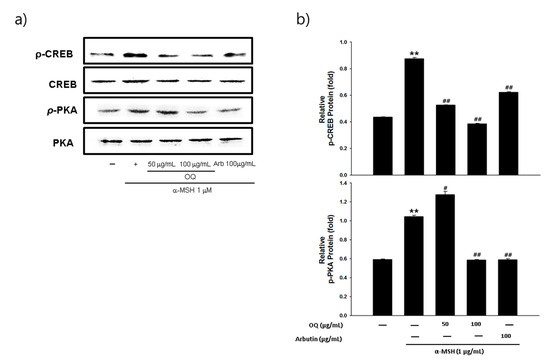
Figure 4. (a) Effects of OQ and arbutin on the p-CREB, CREB, p-PKA, and PKA protein expression in B16F10 cells. B16F10 cells were treated with the indicated concentrations of the OQ and arbutin prior to α-MSH treatment for 3 h. (b) Quantitative analysis of p-CREB, CREB, p-PKA, and PKA by western blotting. Values are significantly different by Duncan’s multiple range test (significant compared to the vehicle-treated control, ** p < 0.01; significant compared to α-MSH, # p < 0.05, ## p < 0.01, bars indicate S.D.).
5. Discussion
Previous studies have reported the isolation and purification of quercetin from plant extracts. However, limited research has been undertaken on the artificial synthesis of quercetin derivatives. Here, we attempted to synthesize a derivative mixture using quercetin and oleyl bromide by adjusting the molar ratio for the first time. We repeatedly succeeded in producing OQ using 1.5 equivalent conditions (Scheme 1). Quercetin has five hydroxyl groups; its B-ring comprises a catechol group and the 5-OH group of the A-ring forms an intramolecular hydrogen bond with the C-4 carbonyl group. It is well known that the hydroxyl group of quercetin has similar reactivity with the 3-position (3-OH), 7-position (7-OH), and 4′-position (4′-OH). These positions have high electron spin densities and acidities, but different steric structures [13][20][21][22]. Therefore, when a nucleophilic substitution reaction is performed using a brominated compound, substitution reactions occur simultaneously at positions 3, 7, and 4′. Unless the reaction conditions are specifically controlled, it is not easy to substitute one or two functional groups in the OH position, except for the 4′position. Previous studies have shown that the hydroxyl group of quercetin occurs gradually in the sequential order of 4′ > 7 > 3 > 3′ > 5 [23]. The catechol group on the B ring (3′-OH and 4′-OH) of quercetin binds to the active site of tyrosinase and acts as an inhibitor. Moreover, other studies have confirmed the alkylation reaction of the quercetin hydroxyl group was 7 > 4′> 3 > 3 > 5 [24]. In the present study, we purified the oleyl derivative of quercetin at positions 3 and 7 by screening for tyrosinase inhibition. Because the hydroxyl group of quercetin has a different reactivity when synthesized depending on the position, most quercetin synthesis has been performed at the desired position after protecting the -OH group. In a previous study, protection was carried out in 3-step, and n-butyl bromide, allyl chloride, cinnamyl chloride, and geranyl bromide were synthesized at the 3rd and 7th positions [25]. Al-Jabban et al. [26] confirmed that the cytotoxic efficacy varied depending on the location of the hydroxyl group synthesized in quercetin. The alkyl group was adjusted to three equivalents and reacted in one step. It was synthesized at positions 3,4′,7 of quercetin, and the derivatives were found to be more effective than quercetin in prostate cancer cells. Kim et al. [27] suggested that the longer the length of the alkyl chain, the better the antioxidant activity. In our study, synthesis was performed using a novel method that has not been used in previous studies. The monomers were fixed at the 3rd and 7th positions of the OH group of quercetin via molar equivalence control. Our findings suggest that a novel whitening agent can be developed by a selective substitution reaction using simple halide compounds such as oleyl bromide. In the future, it is necessary to evaluate the toxicity and whitening of the quercetin derivative synthesized in the form of trimers, and it will be of scientific significance to evaluate the derivative activity using a chloride-leaving group.
For theoretical studies, enzyme kinetics were performed to demonstrate the whitening activity of the new compound using an engineering system. In previous studies, it was reported that the KI value (inhibition constant) and tyrosinase inhibitory concentration of the quercetin derivative with a catechol group in the B ring differed according to the molecular structure of the synthetic material. The tyrosinase inhibitory activity of quercetin was previously described from its ability to chelate copper in the binuclear active center of the enzyme [28]. Chen et al. [15] measured the kinetics of tyrosinase inhibition by quercetin, and was a competitive inhibitor of tyrosinase, with a KI value of 0.0386 mM. Hence, quercetin and OQ inhibit the enzyme competitively, which is a competitive inhibition mechanism. The inhibition of tyrosinase by OQ is a slow and reversible reaction involving the activity of other enzymes, such as quercetin. The kinetic constants of OQ were consistent with those of quercetin, except for Vmax (Table 1). However, there were some discrepancies between the kinetic values of quercetin from different sources. Controversial data have been published using the same raw material as quercetin from different sources [3]. Thus, the source and preparation method of quercetin must be described before concluding the IC50 and parameter values of the tyrosinase enzyme kinetics model. The pyrone moiety is responsible for the inhibitory activity of tyrosinase because it preferentially chelates copper in the enzyme, even if other moieties exist in the same molecule. In the present study, the measured KI value of quercetin was 0.2459 mM. The KI values could be different from those in previous studies because they are affected by the reaction time and substrate and enzyme concentrations. Previous studies have synthesized quercetin-7-oleate using quercetin and oleic acid, and their inhibition of tyrosinase was measured [16]. Quercetin-7-oleate is a competitive inhibitor of tyrosinase. To determine which quercetin derivative is most beneficial in promoting anti-melanogenesis effects, each hydroxy group of the quercetin skeleton was selectively protected.
Melanin is a polymorph and a multifunctional biopolymer. Melanin is synthesized in melanocytes, which are localized in the basal layer of the epidermis. Melanogenesis depends on the regulation of melanogenic proteins, such as tyrosinase, TRP-1, and TRP-2. Many signaling pathways produce melanin, and all signals eventually upregulate MITF. Activated MITF stimulates TRP-1, TRP-2, and tyrosinase expression; therefore, melanin is formed [29]. We investigated the inhibitory effect of OQ on melanogenesis and found that it downregulated tyrosinase, TRP-1, TRP-2, and MITF expression. The regulation mechanism of OQ might be the same as that of quercetin and should be further studied. While the present study focused on tyrosinase, other regulation pathways of melanin would be possible, such as feedback loop regulation and kinase inhibition. Several protein kinases (protein kinase C, Ca/calmodulin-dependent protein kinase, tyrosine kinase, and phosphatidylinositol-3-kinase) present in cells are involved in melanogenesis regulation [30]. The promotion of melanogenesis increases tyrosinase expression by factors that increase cyclic AMP or by post-translational modification of an already existing enzyme [31]. As evidence for the increase in tyrosinase expression, cyclic AMP has been reported to increase the m-RNA expression against tyrosinase [32]. Therefore, OQ reduces intracellular cyclic AMP concentration and tyrosinase via cyclic AMP-dependent protein kinase. To determine at which stage various protein kinases are involved in the signaling pathway of MSH, it is necessary to process PMA, a protein kinase C activator, and confirm it via a downregulation process in the future.
There has been controversy in previous studies over the range of toxicity and whitening activity in quercetin. The anti-melanogenesis effect of quercetin is unclear, and it remains to be determined whether quercetin induces an increase or decrease in melanin content. The effect might depend on the quercetin concentration, which should be tested both in vitro and in vivo. Regarding the controversy over the whitening activity of quercetin, Choi and Shin [3] investigated and reported the concentration range and toxicity range of whitening activity. When the quercetin concentration ranged from 10 to 20 µg/mL, melanin content was increased, and when quercetin concentration ranged from 20 to 50 µg/mL, melanin content decreased in cells. Therefore, quercetin showed strong toxicity even at concentrations below 10 µg/mL and no whitening effect at the concentration level; therefore, quercetin was not suitable as a safe whitening agent. In the present study, synthesis was conducted based on the quercetin structure to develop a new whitening material that was safe and useful when applied to the skin. OQ, the novel compound developed, showed a high whitening effect and did not show cytotoxicity even at concentrations below 100 µg/mL. Previous studies have confirmed the anti-melanogenic activity of β-arbutin, kojic acid, bis(4-hydroxybenzyl)sulfide, and inularin using a zebrafish model. The concentrations that resulted in reduced pigmentation levels compared to the control were 1000, 25, 10, and 10 µM [33][34]. There have been no studies on the anti-melanogenesis activity of zebrafish using quercetin and quercetin derivatives. In the present study, the whitening activity and toxic concentration of quercetin and quercetin derivatives were confirmed through in vitro and in vivo experiments, which were not found in previous studies. To quantitatively analyze tyrosinase inhibition activity, a three-dimensional melanoma cell culture technique for melanin quantification should be used in future research [35].
In some studies, it was found that tyrosinase activity in cell systems was enhanced; however, there was no effect on protein expression, resulting in the overexpression of tyrosinase due to quercetin treatment at concentrations of 1–20 µM [6]. Takekoshi et al. [7] showed an increase in melanin content at quercetin concentrations > 50 µM [7]. Moreover, tyrosinase and TRP-2 were overexpressed at quercetin concentrations of 5–160 µM, but there was no effect on TRP-1 at quercetin concentrations of 50–160 µM. Similarly, the same group showed that at 10 µM quercetin, melanin content increased and tyrosinase was overexpressed after 3 days [8]. The efficacy of quercetin derivatives as cosmetic ingredients is somewhat certain; however, further studies on the synthesis of various compound libraries and molecular signaling should be undertaken using suitable materials and methods.
References
- Taieb, A.; Cario-Andre, M.; Briganti, S.; Picardo, M. Inhibitors and enhancers of melanogenesis. In Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions; Borovansky, J., Riley, P.A., Eds.; Wiley-Blackwell: Weinheim, Germany, 2011; Volume 5, pp. 117–166.
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571.
- Choi, M.H.; Shin, H.J. Anti-melanogenesis effect of quercetin. Cosmetics 2016, 3, 18.
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27.
- Sugimoto, K.; Nomura, K.; Nishimura, T.; Kiso, T.; Sugimoto, K.; Kuriki, T. Syntheses of α-arbutin-α-glycosides and their inhibitory effects on human tyrosinase. J. Biosci. Bioeng. 2005, 99, 272–276.
- Nagata, H.; Takekoshi, S.; Takeyama, R.; Homma, T.; Osamura, R.Y. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and normal human melanocytes. Pigment Cell Res. 2004, 17, 66–73.
- Takekoshi, S.; Matsuzaki, K.; Kitatani, K. Quercetin stimulates melanogenesis in hair follicle melanocyte of the mouse. Tokai J. Exp. Clin. Med. 2013, 38, 129–134.
- Takekoshi, S.; Nagata, H.; Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med. 2014, 39, 116–121.
- Arung, E.T.; Kusuma, I.W.; Shimizu, K.; Kondo, R. Tyrosinase inhibitory effect of quercetin 4′-O-b-D-glucopyranoside from dried skin of red onion (Allium cepa). Nat. Prod. Res. 2011, 25, 256–263.
- Strzepek-Gomolka, M.; Gawel-Beben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Glowniak, K.; Kukula-Koch, W. Identification of mushroom and murine tyrosinase inhibitors from Achillea biebersteinii Afan. extract. Molecules 2021, 26, 964.
- Chao, H.C.; Najjaa, H.; Villareal, M.O.; Ksouri, R.; Han, J.; Neffati, M.; Isoda, H. Arthrophytumscoparium inhibits melanogenesis through the down-regulation of tyrosinase and melanogenic gene expressions in B16 melanoma cells. Exp. Dermatol. 2013, 22, 131–136.
- Taira, J.; Tsuchida, E.; Uehara, M.; Ohhama, N.; Ohmine, W.; Ogi, T. The leaf extract of Mallotus japonicas and its major active constituent, rutin, suppressed on melanin production in murine B16F1 melanoma. Asian Pac. J. Trop. Biomed. 2015, 5, 819–823.
- Kato, K.; Ninomiya, M.; Tanaka, K.; Koketsu, M. Effects of functional groups and sugar composition of quercetin derivatives on their radical scavenging properties. J. Nat. Prod. 2016, 79, 1808–1814.
- Lee, M.K.; Moon, S.S.; Lee, S.E.; Bok, S.H.; Jeong, T.S.; Park, Y.B.; Choi, M.S. Naringenin 7-O-cetyl ether as inhibitor of HMG-CoA reductase and modulator of plasma and hepatic lipids in high cholesterol-fed rats. Bioorg. Med. Chem. 2003, 11, 393–398.
- Chen, Q.X.; Kubo, I. Kinetics of mushroom tyrosinase inhibition by quercetin. J. Agric. Food Chem. 2002, 50, 4108–4112.
- Vaezi, M.; Behbehani, G.R.; Gheibi, N.; Farasat, A. Thermodynamic, kinetic and docking studies of some unsaturated fatty acids-quercetin derivatives as inhibitors of mushroom tyrosinase. AIMS Biophys. 2020, 7, 393–410.
- Yan, J.; Zhang, G.; Hu, Y.; Ma, Y. Effect of luteolin on xanthine oxidase: Inhibition kinetics and interaction mechanism merging with docking simulation. Food Chem. 2013, 141, 3766–3773.
- Tsou, C.L. Kinetics of substrate reaction during irreversible modification of enzyme activity. In Advances in Enzymology and Related Areas of Molecular Biology; Meister, A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1988; Volume 61, pp. 381–436.
- Oh, E.J.; Park, J.I.; Lee, J.E.; Myung, C.H.; Kim, S.Y.; Chang, S.E.; Hwang, J.S. A novel role of serotonin receptor 2B agonist as an anti-melanogenesis agent. Int. J. Mol. Sci. 2016, 17, 546.
- Trouillas, P.; Marsal, P.; Siri, D.; Lazzaroni, R.; Duroux, J.L. A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: The specificity of the 3-OH site. Food Chem. 2006, 97, 679–688.
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709.
- Lee, H.J.; Kerr, R.A.; Korshavn, K.J.; Lee, J.; Kang, J.; Ramamoorthy, A.; Ruotolo, B.T.; Lim, M.H. Effects of hydroxyl group variations on a flavonoid backbone toward modulation of metal-free and metal-induced amyloid-β aggregation. Inorg. Chem. Front. 2016, 3, 381–392.
- Rao, K.V.; Owoyale, J.A. Partial methylation of quercetin: Direct synthesis of tamarixetin, ombuin and ayanin. J. Heterocycl. Chem. 1976, 13, 1293–1295.
- Zhou, Z.H.; Fang, Z.; Jin, H.; Chen, Y.; He, L. Selective monomethylation of quercetin. Synthesis 2010, 23, 3980–3986.
- Bao, X.R.; Liao, H.; Qu, J.; Sun, Y.; Guo, X.; Wang, E.X.; Zhen, Y.H. Synthesis, characterization and cytotoxicity of alkylated quercetin derivatives. Iran. J. Pharm. Res. 2016, 15, 329.
- Al-Jabban, S.M.; Zhang, X.; Chen, G.; Mekuria, E.A.; Rakotondraibe, L.H.; Chen, Q.H. Synthesis and anti-proliferative effects of quercetin derivatives. Nat. Prod. Commun. 2015, 10, 2113–2118.
- Kim, M.; Park, Y.; Cho, S.; Burapan, S.; Han, J. Synthesis of alkyl quercetin derivatives. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 343–348.
- Wilcox, D.E.; Porras, A.G.; Hwang, Y.T.; Lerch, K.; Winkler, M.E.; Solomon, E.I. Substrate analog binding to the coupled binuclear copper active site in tyrosinase. J. Am. Chem. Soc. 1985, 107, 4015–4027.
- Yu, Q.; Fan, L.; Duan, Z. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food Chem. 2019, 297, 124910.
- Fuller, B.B.; Lunsford, J.B.; Iman, D.S. Alpha-melanocyte-stimulating hormone regulation of tyrosinase in Cloudman S-91 mouse melanoma cell cultures. J. Biol. Chem. 1987, 262, 4024–4033.
- Park, H.Y.; Russakovsky, V.; Ao, Y.; Fernandez, E.; Gilchrest, B.A. α-melanocyte stimulating hormone-induced pigmentation is blocked by depletion of protein kinase C. Exp. Cell Res. 1996, 227, 70–79.
- Kuzumaki, T.; Matsuda, A.; Wakamatsu, K.; Ito, S.; Ishikawa, K. Eumelanin biosynthesis is regulated by coordinate expression of tyrosinase and tyrosinase-related protein-1 genes. Exp. Cell Res. 1993, 207, 33–40.
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 1–8.
- Jang, D.K.; Jung, S.H.; Jeong, J.H.; Yoo, H.M.; Lee, I.S.; Shin, H.S. The antimelanogenic effect of inularin isolated from flowers of Inula britannica on B16F10 melanoma cells and zebrafish embryos. J. Microbiol. Biotechnol. 2020, 30, 749–752.
- Chung, S.; Lim, G.J.; Lee, J.Y. Quantitative analysis of melanin content in a three-dimensional melanoma cell culture. Sci. Rep. 2019, 9, 1–9.




