
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Susan L Lindsay | + 1161 word(s) | 1161 | 2021-04-27 11:25:06 | | | |
| 2 | Vicky Zhou | + 2 word(s) | 1163 | 2021-05-05 17:59:53 | | |
Video Upload Options
Mesenchymal stem/stromal cells (MSCs) for transplant-mediated repair represents an important and promising therapeutic strategy after spinal cord injury (SCI). MSCs can be harvested from a wide range of human tissues, however it is likely certain niches are more suited for SCI repair due to their intrinsic capabilities.
1. Introduction
Spinal cord injury (SCI) results in devastating loss of sensory, motor, and autonomic function which leaves a sufferer incapacitated, bound to a wheelchair, and robbed of independence. SCI is therefore associated with substantial levels of suffering and a high socioeconomic burden. Clinical outcomes depend on the severity and location of the lesion but may include partial or complete loss of function below the level of injury. Trauma, the most common cause of SCI in the Western world, results in a complex injury pathology. The initial mechanical insult causes haemorrhage, shearing of axons, destruction of cells, and triggers a cascade of secondary injury mechanisms that lead to further cell death and demyelination [1]. Injury triggers an immune response, the formation of a glial scar, and, over the longer term, development of fluid filled cystic cavities [2][3]. As a result, the loss of function after SCI is generally permanent, and since there are currently no effective treatments, it represents a major unmet clinical need.
There are numerous strategies currently reported for the treatment of SCI; however, stem cell transplantation has gained the most interest [4][5]. The transplantation of autologous or allogeneic cells, including differentiated glia and various stem cells, have been extensively explored [6][7][8][9][10]. The aim of cellular transplantation is that it creates an environment favourable to repair, one which may offer neuroprotection, immune regulation, eventual axonal regeneration, neuronal circuit formation, and myelin regeneration [11][12][13]. One popular cellular candidate are mesenchymal stromal cells (MSCs). MSCs can be isolated from numerous tissue sources, are relatively easy to expand in vitro, and have unique beneficial immunological properties [14]. Furthermore, the MSC secretome, which has a paracrine effect on the local environment after injury, have made them well suited to SCI repair (Figure 1). MSCs were first identified in 1968 by Friedenstein and colleagues as a population of adherent cells present in the bone marrow (BM) which exhibited fibroblast-like morphology [15][16]. Today, numerous other tissue sources have been identified, such as adipose, dental pulp, periodontal ligament, tendon, skin, muscle, and newer tissues, such as the olfactory mucosa and lung [17][18]. There are likely many other sources within the human body; however, practical limitations rule them out as potential therapeutic sources [18][19]. MSCs are defined by their capability to self-renew, ability to differentiate into three specific cell lineages in vitro (osteoblasts, adipocytes, and chondrocytes; although in vivo they can make other stromal cell types, including endothelial cells) and by their expression of a subset of cell surface proteins; a specific MSC marker has yet to be identified [20]. The International Society for Cellular Therapy has stated that MSCs should express CD105, CD73, and CD90 and lack expression of CD45, CD34, CD14, CD11b, CD79a, or CD19, and human leukocyte antigen-DR (HLA-DR) surface molecules.
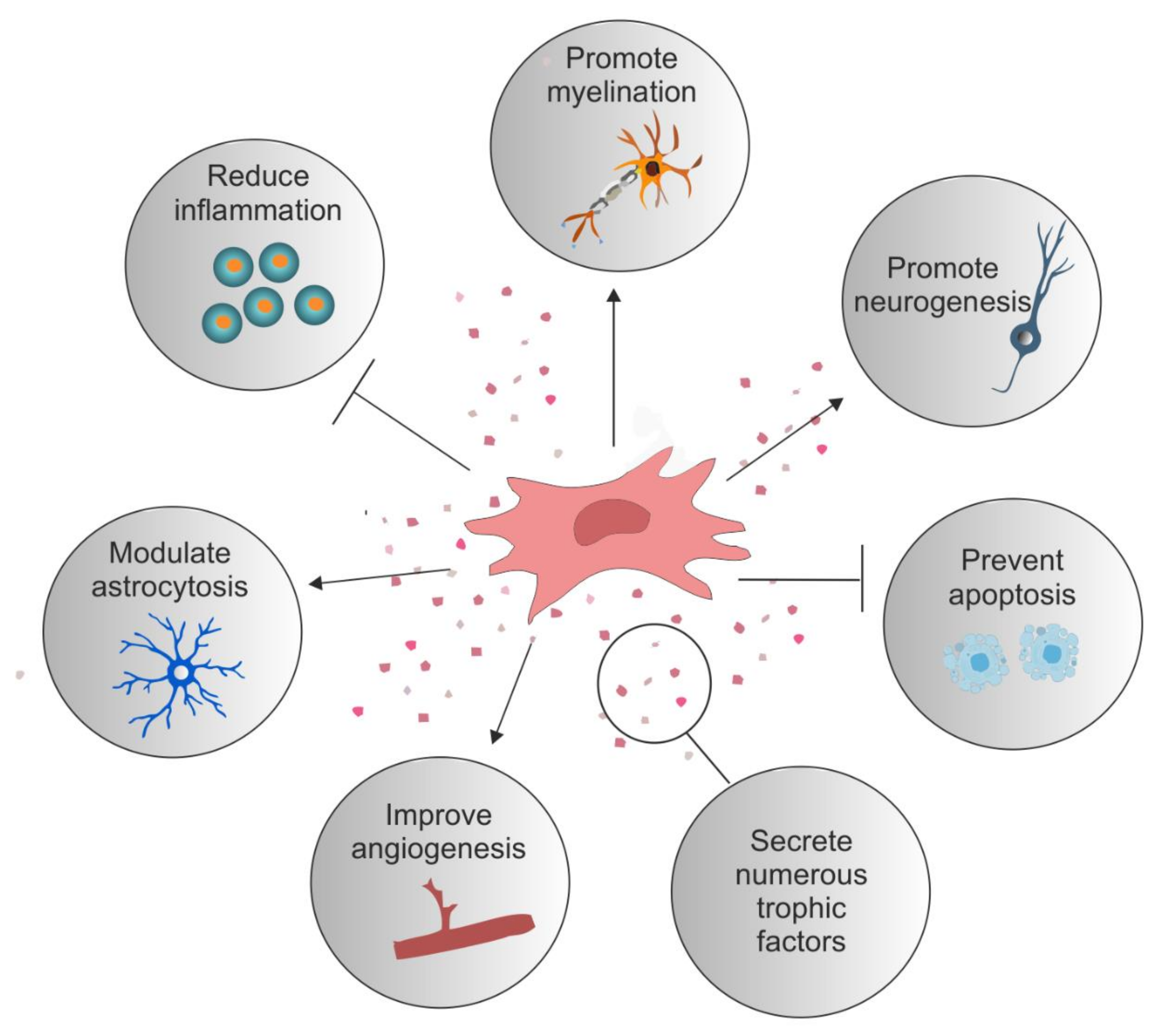
Figure 1. Reparative role of mesenchymal stromal cells. Examples of the reparative potential of mesenchymal stromal cells (MSCs) for spinal cord injury (SCI). MSCs secrete numerous trophic factors and anti-inflammatory molecules that change the injury milieu to pro-regenerative. These secreted factors have an anti-inflammatory effect on numerous immune cells such as T cells, B cells, macrophages, and microglia. They reduce astrocytosis and promote axonal growth and neuroprotection. They stimulate angiogenesis and offer protection against apoptotic cell death. Certain MSC types promote the differentiation of oligodendrocytes and myelination.
There is a wealth of literature describing the promising effects of MSC therapy after SCI. In general, they have both an anti-inflammatory and pro-regenerative capacity in human and animal models, which has been covered extensively in other recent reviews [21][22]. Importantly, their use in phase I/II clinical SCI trials have confirmed their safety [21][23][24][25]. However, whilst many clinical trials report promising efficacy for sensorimotor function, the risk of bias is high since few studies have included control groups, and the study subject number is low [24]. Many aspects concerning MSC therapy require clearer definition if they are to be fully translated to the clinic. For example, MSCs derived from specific cellular niches may have potential limitations due to niche-specific inherent properties. Understanding the MSC role both within their resident niche and their fate after SCI will improve their use as a therapy. Tissue-specific stem cells support the tissue type from which they originate, meaning that specific MSC types might be more suited for the treatment of SCI than others. In addition, the ease of accessibility of the anatomical location of specific MSC types may be more practical for clinical translation. Crucially, the generation of MSCs will need to follow strict good manufacturing practice (GMP) guidelines to ensure the safety and quality of the end-product before use in clinical trials. Therefore, culture reagents used in their isolation and maintenance must meet these guidelines, and the impact of changing protocols to one more GMP compliant should be considered.
2. Therapeutic Potential of Niche-Specific Mesenchymal Stromal Cells for Spinal Cord Injury Repair
An ideal MSC candidate for transplant-mediated repair would be one that is able to modulate the inflammatory environment, promote myelination, have neurogenic properties, and preferably reside in a niche that is unaffected by the injury itself. In addition, cells that are easily harvested and grown quickly to clinically relevant numbers offer clear advantages. A targeted approach to the isolation of niche-specific MSCs more intrinsically suited for the treatment of SCI may offer better therapeutic benefits (Figure 2, Table 1).
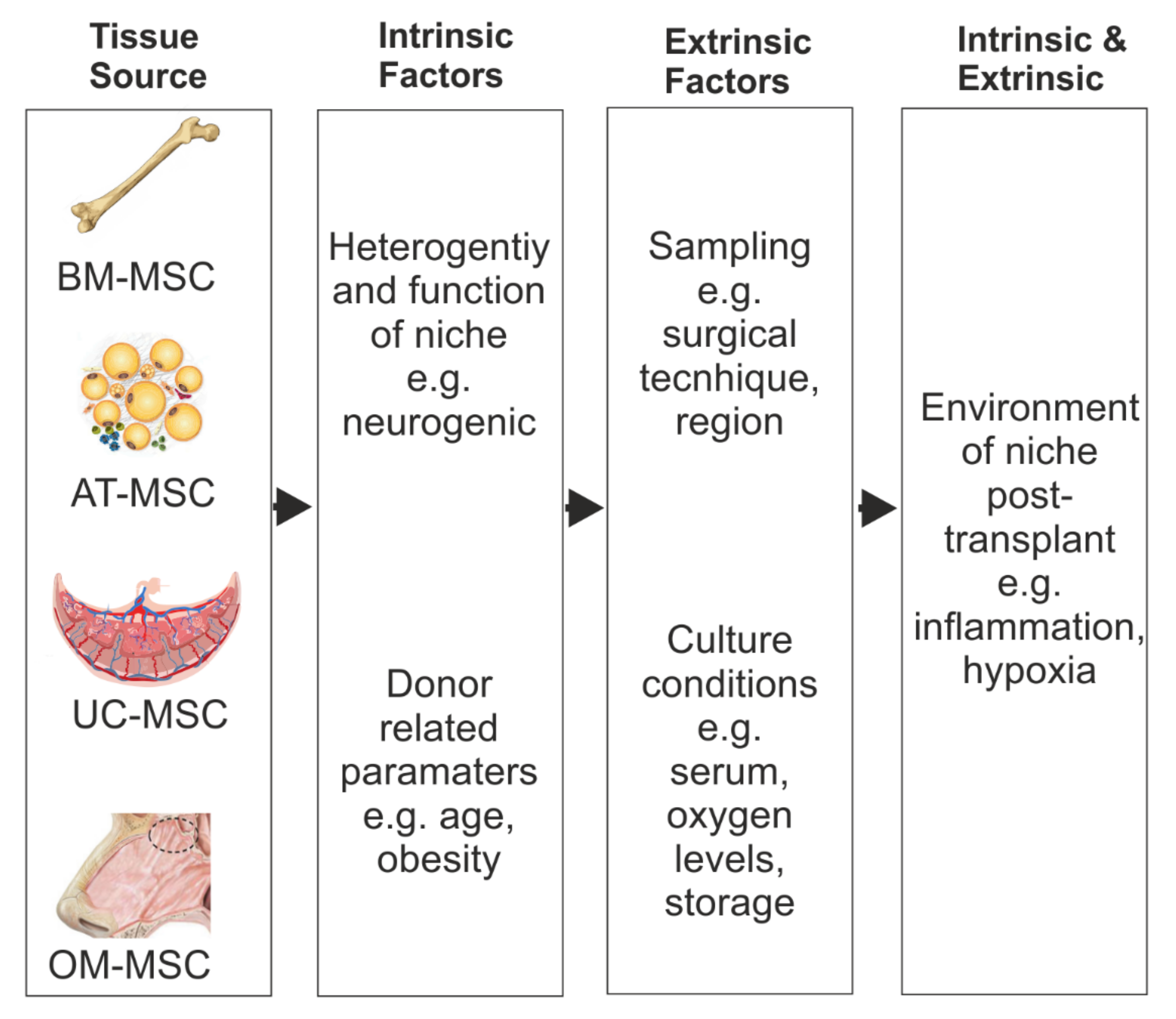
Figure 2. Factors which can affect MSC repair benefits. Schematic representation of the potential MSC heterogeneity because of their different tissue source and the intrinsic and extrinsic factors that could influence their repair benefits after SCI.
Table 1. Comparison of key characteristics of MSCs harvested from different sources.
| BM-MSC | AD-MSC | UC-MSC | OM-MSC | |
|---|---|---|---|---|
| Niche | Haematopoietic | Angiogenic | Haematopoietic | Neurogenic |
| Tissue Availability | ++ | +++ | +++ | +++ |
| Use in SCI clinical trials | +++ | ++ | +++ | + |
| Procedure | Invasive | Minimally Invasive | Not Invasive | Minimally Invasive |
| Proliferative Capacity | + | ++ | +++ | +++ |
| Cell Yield | + | +++ | ++ | +++ |
| Autologous use | +++ | +++ | + | +++ |
| Allogenic use | +++ | +++ | +++ | + |
| Nestin expression | ++ | + | ++ | +++ |
Different niche-derived MSCs may offer differing therapeutic benefits. BM-, AD-, and UC-derived MSCs have all reached clinical trial for the treatment of SCI across the world. Although olfactory tissue and cells have also reached clinical trial, purified OM-MSCs have yet to be tested. Consideration should be given to the impact of the procedure on the patient donor and whether enough cells will be generated quickly post tissue acquisition. Nestin expression on MSCs correlates with enhanced CXCL12 expression, which promotes myelination. This may be an important marker for isolating MSCs with repair characteristics better suited for SCI repair. Within the BM there is a small population of Nestin+ MSCs; however, these may be difficult to isolate. UC-MSCs express high levels of nestin soon after isolation, although this is lost after passage. AD-MSCs have not been found to express nestin but can upregulate it after neural differentiation in vitro. OM-MSCs constitutively express nestin which is not lost during passage. Key: +++ high, ++ moderate, + low.
References
- Norenberg, M.D.; Smith, J.; Marcillo, A. The Pathology of Human Spinal Cord Injury: Defining the Problems. J. Neurotrauma 2004, 21, 429–440.
- Brennan, F.H.; Popovich, P.G. Emerging targets for reprograming the immune response to promote repair and recovery of function after spinal cord injury. Curr. Opin. Neurol. 2018, 31, 334–344.
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391.
- Tetzlaff, W.; Okon, E.B.; Karimi-Abdolrezaee, S.; Hill, C.E.; Sparling, J.S.; Plemel, J.R.; Plunet, W.T.; Tsai, E.C.; Baptiste, D.; Smithson, L.J.; et al. A Systematic Review of Cellular Transplantation Therapies for Spinal Cord Injury. J. Neurotrauma 2011, 28, 1611–1682.
- Zavvarian, M.-M.; Toossi, A.; Khazaei, M.; Hong, J.; Fehlings, M. Novel innovations in cell and gene therapies for spinal cord injury. F1000Research 2020, 9, 279.
- Biernaskie, J.; Sparling, J.S.; Liu, J.; Shannon, C.P.; Plemel, J.R.; Xie, Y.; Miller, F.D.; Tetzlaff, W. Skin-Derived Precursors Generate Myelinating Schwann Cells That Promote Remyelination and Functional Recovery after Contusion Spinal Cord Injury. J. Neurosci. 2007, 27, 9545–9559.
- Sparling, J.S.; Bretzner, F.; Biernaskie, J.; Assinck, P.; Jiang, Y.; Arisato, H.; Plunet, W.T.; Borisoff, J.; Liu, J.; Miller, F.D.; et al. Schwann Cells Generated from Neonatal Skin-Derived Precursors or Neonatal Peripheral Nerve Improve Functional Recovery after Acute Transplantation into the Partially Injured Cervical Spinal Cord of the Rat. J. Neurosci. 2015, 35, 6714–6730.
- Toft, A.; Scott, D.T.; Barnett, S.C.; Riddell, J.S. Electrophysiological evidence that olfactory cell transplants improve function after spinal cord injury. Brain J. Neurol. 2006, 130, 970–984.
- Barnett, S.C.; Riddell, J.S. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair—What can it achieve? Nature clinical practice. Neurology 2007, 3, 152–161.
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; A Salegio, E.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490.
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647.
- Hunt, M.; Lu, P.; Tuszynski, M.H. Myelination of axons emerging from neural progenitor grafts after spinal cord injury. Exp. Neurol. 2017, 296, 69–73.
- Faulkner, J.; Keirstead, H.S. Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl. Immunol. 2005, 15, 131–142.
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22.
- Friedenstein, A.J.; Petrakova, K.V.; I Kurolesova, A.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247.
- Friedenstein, A.J.; Deriglasova, U.F.; Kulagina, N.N.; Panasuk, A.F.; Rudakowa, S.F.; Luriá, E.A.; Ruadkow, I.A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974, 2, 83–92.
- Lindsay, S.L.; Barnett, S.C. Are nestin-positive mesenchymal stromal cells a better source of cells for CNS repair? Neurochem. Int. 2017, 106, 101–107.
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119 Pt 11, 2204–2213.
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med J. 2018, 18, e264–e277.
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147.
- Johnson, L.; Pickard, M.; Johnson, W. The Comparative Effects of Mesenchymal Stem Cell Transplantation Therapy for Spinal Cord Injury in Humans and Animal Models: A Systematic Review and Meta-Analysis. Biology 2021, 10, 230.
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021, 30.
- Mendonça, M.V.P.; LaRocca, T.F.; de Freitas Souza, B.S.; Villarreal, C.F.; Silva, L.F.M.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.P.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126.
- Hur, J.W.; Cho, T.-H.; Park, D.-H.; Lee, J.-B.; Park, J.-Y.; Chung, Y.-G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2015, 39, 655–664.
- Willison, A.G.; Smith, S.; Davies, B.M.; Kotter, M.R.N.; Barnett, S.C. A scoping review of trials for cell-based therapies in human spinal cord injury. Spinal Cord 2020, 58, 844–856.




