Modulation of macrophage plasticity is emerging as a successful strategy in tissue engineering (TE) to control the immune response elicited by the implanted material. Indeed, one major determinant of success in regenerating tissues and organs is to achieve the correct balance between immune pro-inflammatory and pro-resolution players. In recent years, nanoparticle-mediated macrophage polarization towards the pro- or anti-inflammatory subtypes is gaining increasing interest in the biomedical field. In TE, despite significant progress in the use of nanomaterials, the full potential of nanoparticles as effective immunomodulators has not yet been completely realized. This work discusses the contribution that bioactive inorganic nanoparticles may give to TE applications, helping native or synthetic scaffolds to direct macrophage polarization for skeletal muscle regeneration.
- nanotechnology
- tissue engineering
- immunomodulation
- macrophage plasticity
- skeletal muscle regeneration
1. Introduction
Tissue engineering (TE) is a multidisciplinary field including bio-medicine, material science, and engineering, aimed at manufacturing functional biological tissues to be implanted in organs damaged by otherwise incurable diseases or severe casualties [1][2]. Engineered tissues are also fundamental to set up in vitro models of human physiological systems, replacing animal models for drug development, toxicology studies, etc. [3].
A successful TE treatment depends on the immune response of the recipient tissue. In fact, the engineered tissue must initially challenge the inflammatory microenvironment of the damaged tissue to establish an efficient integration and, after the implantation, the inflammatory reaction characterizes a possible rejection process. The traditional strategy adopted after tissue and organ transplantation aims at minimizing the host immune response through anti-inflammatory and immunosuppressive therapies. However, if, on one hand, the immune system is often the cause of implants rejection, on the other, several immune components positively affect tissue regeneration and healing [4]. Therefore, controlling the balance between immune pro-inflammatory and pro-resolution players represents a better strategy to ensure implant tolerance than suppressing the immune response [5].
Among the immune cells involved in the foreign body response, macrophages play a central role. Macrophages are effector immune cells simplistically divided into two classes, named M1 and M2. To ensure regeneration, a balance between M1 and M2 activities (pro- and anti-inflammatory, respectively) shifting over time is required [6]; hence, scaffold-based approaches to direct macrophage polarization are gaining much interest in TE for regenerative medicine.
Nanomaterials are emerging as effective agents able to target macrophages, perturbing their polarization and thus their activity [7]. As a consequence, in recent years, optimally designed-nanoparticles (NPs) for the modulation of macrophage plasticity have been studied for treating diseases characterized by hyper-tolerant or inflammatory immune microenvironment, such as cancer [8] and inflammatory diseases [9], respectively. As far as TE is concerned, the full potential of nanoparticles as macrophage regulators has not yet been fully realized. This review aims at discussing nanotechnology impact on TE in terms of directing macrophage polarization and reprogramming, focusing on bioactive inorganic nanoparticles prospects for skeletal muscle regeneration.
2. Nanoparticles to Direct Macrophage Polarization
Nanoparticles (NPs) have been initially developed to overcome problems of bioavailability, body retention, solubility, stability, and selectivity of pharmaceutical agents, protecting the carried drug until reaching the desired body district. However, nanomaterials at the nanoscale (1–100 nm) acquired peculiar properties, due to the increased reactive surface/bulk ratio with respect to micro- and macro-structures [10], making them attractive for TE.
Indeed, in the 2000s, a key role has been recognized for nanomaterials in TE, as nanocomposite polymers, both in the form of electrospun fibers and hydrogels, often provide superior mechanical, functional, and electrical properties [11]. In the context of skeletal muscle regeneration, for example, aligned nanofibrous scaffolds (e.g., PCL/collagen) favored cell alignment and myotube formation, thus promoting muscle regeneration [12].
It is noteworthy that the role of NPs as modulators of macrophage plasticity is strongly emerging; indeed, due to their particulate (instead of molecular) nature, nanoparticles preferentially target professional phagocytes, such as macrophages [13]. This property is of a paramount importance for the success of TE procedures. Therefore, several research works focused on the use of organic nanovesicles (liposomes, polysaccharides, capsules, etc.) carrying encapsulated bioactive molecules [14], such as flavonoids [15], miRs, and cytokines [16], in order to regulate macrophage activity. In this study, instead, we investigate three major inorganic NPs (gold, titanium oxide, and cerium oxide NPs, later denoted as AuNPs, TiO2 NPs, and CeO2 NPs), not only being exploitable as carriers for drugs and molecules, but also being characterized by intrinsic bioactivity, which renders them promising candidates for TE therapies (Figure 2, Table 1).
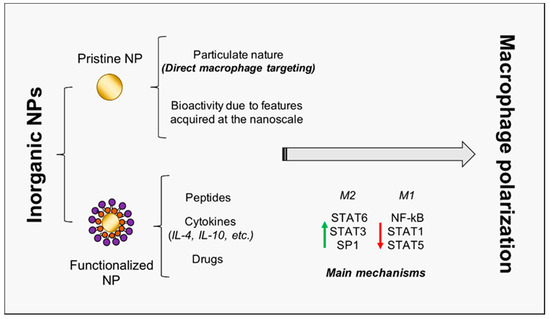
Figure 2. Inorganic NP-mediated macrophage polarization towards regeneration. The schematic representation of inorganic NPs double role is reported. NPs may act as pristine NPs, characterized by intrinsic bioactivity, and as carriers for peptides, cytokines, and drugs. Principal transcription factors involved in macrophage activation and polarization are also included. NP = nanoparticle; IL = interleukin; STAT = signal transducer and activator of transcription; NF-kB = nuclear factor kappa-light-chain-enhancer of activated B cells.
Table 1. Nanoparticle-induced macrophage polarization towards M2 phenotype.
| NPs | Shape | Size (nm) |
Surface Modification | Initial Phenotype | Polarized Phenotype | Model | Used Markers | Clinical Aim | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Au | rods | ND | PEGylation + RGD | M∅ | M2 | Mouse | Arg-1, IL-4, TNF-α, Retnla | Acute hepatitis | [17] |
| spheres | 13 | hexapeptides | M2 | M2 | Mouse BMDMs | IL-12, IL-6, IL-10, iNOS, Arg-1, YM1 | Acute lung injury | [18] | |
| M1 | M2 | ||||||||
| M2 | ALI Mouse model | CD80, CD206 | |||||||
| spheres | 30 | PEGylation + IL-4 | M∅ | M2 | Human THP-1 cell line | CD206, CD163, IL-4 | Muscle recovery | [19] | |
| 30 | PEGylation + IL-4 | M∅ | M2 | Mouse | CD206, CD80 | ||||
| 100 | None | M∅ | M1 | Human THP-1 cell line | CD206, CD163, IL-4 | ||||
| TiO2 | tubes | 110 | None | M∅ | M1 | Mouse RAW 264.7 cell line | CCR7, IL-6, IL-10, VEGF, BMP2, TGF-β1 | Osteogenesis | [20] |
| M∅ | M2 | Mouse RAW 264.7 cell line + MSCs | |||||||
| disks | 10 * | Ca2+, Sr2+ | M∅ | M2 | Mouse RAW 264.7 cell line | Arg-1, CD163, CD86, iNOS | Osteogenesis | [21] | |
| tubes | 65 92 142 |
None | M∅ | M2 | Human THP-1 cell line | Arg-1, CD206, IL-10, VEGF | Heart valve replacement | [22] | |
| CeO2 | thin layer | ND | None | M∅ | M2 | Mouse RAW 264.7 cell line | CD163, CD206, IL-6, TNF-α, IL-10, TGF-β1 | Osteogenesis | [23] |
| thin layer | ND | None | M∅ | M2 | Mouse RAW 264.7 cell line + BMSCs | CCR7, CD206, TNF-α, IL-10 | Osteogenesis | [24] | |
| ND | None | Rat |
NPs = nanoparticles; RGD = arginine-glycine-aspartic acid; M = macrophage; Arg-1 = arginase-1; IL = interleukin; TNF-α = tumor necrosis factor-α; MSCs = mesenchymal stem cells; BMDM = bone marrow derived macrophages; CCR7 = C-C chemokine receptor 7; VEGF = vascular endothelial growth factor; BMP2 = bone morphogenetic protein 2; TGF-β1 = transforming growth factor β1; NOS = nitric oxide synthase; BMSCs = bone mesenchymal stem cells; * Average surface roughness; ND = not determined.
2.1. Harnessing Gold Nanoparticles to Trigger Skeletal Muscle Regeneration
AuNPs have been extensively studied [25], particularly for cardiac tissue regeneration, due to their ability to enhance scaffold conductivity [11]. Furthermore, they were recently found to improve osteogenic differentiation of human bone marrow-derived mesenchymal stem cells [26]. As regards their immunomodulatory activity, a recent work by Ni highlighted the ability of AuNPs to generate a microenvironment with constraint inflammatory and reparative cytokines [27]. In particular, when facing inflammation upon stimulation with LPS, murine macrophages exposed to AuNPs showed decreased pro-inflammatory and enhanced anti-inflammatory markers, with respect to control cells.
The surface chemistry of AuNPs also allows rapid functionalization with bioactive molecules, making them suitable for several purposes. In the context of macrophage polarization, AuNPs functionalized with the peptide arginine-glycine-aspartic acid (RGD), a motif responsible for cell adhesion to ECM, were observed to dramatically decrease M1 markers expression, while increasing the M2 ones, in macrophages isolated from mouse livers [17]. Furthermore, when coated with the hexapeptides Cys-Leu-Pro-Phe-Phe-Asp, able to inhibit LPS-induced activation of TLR4, AuNPs drove mouse bone marrow-derived macrophages towards the M2 phenotype, both in vitro and in vivo [18]. Cytokines may also be conjugated on nanoparticles surface; a recent work by Raimondo and Mooney, reported that IL-4-conjugated AuNPs recovered injured murine skeletal muscle via NP-directed M2 macrophage polarization [19]; indeed, the authors finely demonstrated that macrophage depletion abrogated the therapeutic effect triggered by the functionalized nanoparticles. The potential positive contribution of AuNPs in skeletal muscle regeneration is confirmed by Ge and colleagues. Indeed, they reported that AuNPs significantly enhanced myogenic differentiation of myoblasts, via activation of the p38 mitogen-activated protein kinase (p38 MAPK) signaling pathway, finally promoting in vivo regeneration in muscle defect models of rats [28].
2.2. Titanium Oxide NPs: From Osteogenesis to Muscle Regeneration?
TiO2 NPs are of significant concern in TE, especially for hard tissue regeneration, because titanium resists corrosion from the body. However, nanometer-thin TiO2 deposition has also been applied for muscle regeneration, significantly enhancing rat skeletal muscle cell attachment and proliferation [29]. Unlike other ceramic NPs, which induced an inflammatory phenotype in primary macrophages, titanium oxide NPs were reported not to foster inflammation [30]. A recent work demonstrated that TiO2 nanotubes promoted osteogenesis via mediating the crosstalk between macrophages and mesenchymal stem cells (MSCs) under oxidative stress, reducing early inflammation by accelerating macrophages M1 to M2 transition [20]. A previous work by Lee, also reported increased M2 subtypes markers (Arg1, MR and CD163) on calcium and strontium (Ca and Sr, respectively)-modified titanium implants, leading to enhanced osteogenesis [21]. Furthermore, TiO2 nanotubes, inducing macrophage elongation and polarization towards the M2 anti-inflammatory state, also promoted endothelialization [22]. In particular, THP-1 cells, when exposed to TiO2 nanotubes, polarized to M2 macrophages and produced high levels of VEGF, which promoted human umbilical vein endothelial cells (HUVEC) proliferation via two main proliferative pathways: the phosphatidylinositol 3-kinase/AKT and the extracellular signal-regulated kinase 1/2 pathways. As vasculature is needed to facilitate construct engraftment and provide nutrient and waste exchange to the regenerating tissue, TiO2 nanoparticles may be able to strongly affect the whole foreign body response, addressing it towards regeneration.
2.3. Cerium Oxide NPs: Not Just Antioxidants
CeO2 NPs are bioactive nanoparticles attracting great interest in the biomedical field, mainly for their strong antioxidant activity, due to the presence on their surface of cerium atoms in both of the oxidation states: Ce3+ and Ce4+ [10]. Besides their valence in cancer research [31], they have recently been emerging as promoters of tissue regeneration. In 2013, topical application of CeO2 NPs was reported to accelerate the healing of dermal wounds in mice, enhancing proliferation and migration of fibroblasts, keratinocytes, and vascular endothelial cells [32]. More recently, CeO2 NPs incorporated into hydroxyapatite coating, via the plasma spraying technique, induced polarization of murine RAW264.7 macrophages towards the M2 phenotype, as demonstrated by the upregulation of the M2 surface markers CD163 and CD206, promoting an osteogenic behavior of bone mesenchymal stem cells (BMSCs) [23]. The improved osteogenic differentiation ability of BMSCs was further found to be proportional to the surface Ce4+/Ce3+ ratio; indeed, the higher ratio achieved better osseointegration in vivo, and enhanced murine macrophages polarization towards the M2 phenotype, increasing the levels of anti-inflammatory cytokines [24].
It is noteworthy that CeO2 NPs demonstrated reliable biocompatibility [33]. This is of a paramount importance; indeed, in general, pharmacological administration of inorganic nanoparticles causes safety issues to arise, rendering nanotoxicology [34] a key discipline for nanoparticles use in biomedicine and TE.
3. Conclusions
Despite the significant progress in TE applications, nanotechnology (both in terms of nanocomposite polymers and/or nanopatterned scaffolds) has been mainly applied to provide scaffolds with superior mechanical, functional, and electrical properties, and to favor muscle cell alignment and differentiation. Nevertheless, as thoroughly discussed in this entry, particular attention should be paid to the induced immune response, especially to macrophage polarization. Indeed, a correct balance between pro- and anti-inflammatory factors is crucial for scaffold engraftment and tissue regeneration. In this context, surprisingly, there is still too little effort to investigate the contribution that nanotechnology may give to direct macrophage polarization, while, in other areas of biomedical research, such as inflammatory diseases and tumors, several bioactive inorganic nanoparticles have been recognized as valuable modulators of macrophage plasticity.
The analysis of the literature performed in this entry unveiled the unpresented potential of Au, TiO2, and CeO2 nanoparticles to effectively modulate macrophage polarization and strongly support TE for regenerative medicine. While AuNPs have already been studied in the field of muscle regeneration, obtaining excellent results both in terms of NP-elicited macrophage polarization and promotion of myogenesis, TiO2, and CeO2 NPs, were mainly tested in the field of osteogenesis, but the results summarized here represent good premises for their immediate application also in skeletal muscle regeneration.
References
- Atala, A. Engineering tissues, organs and cells. J. Tissue Eng. Regen. Med. 2007, 1, 83–96.
- Armstrong, L.; Lako, M.; Buckley, N.; Lappin, T.; Murphy, M.; Nolta, J.; Pittenger, M.; Stojkovic, M. Editorial: Our top 10 developments in stem cell biology over the last 30 years. Stem Cells 2011, 30, 2–9.
- Watson, D.; Hunziker, R.; Wikswo, J. Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology. Exp. Biol. Med. 2017, 242, 1559–1572.
- Crupi, A.; Costa, A.; Tarnok, A.; Melzer, S.; Teodori, L. Inflammation in tissue engineering: The Janus between engraftment and rejection. Eur. J. Immunol. 2015, 45, 3222–3236.
- Carotenuto, F.; Teodori, L.; Maccari, A.; Delbono, L.; Orlando, G.; Di Nardo, P. Turning regenerative technologies into treatment to repair myocardial injuries. J. Cell Mol. Med. 2019, 24, 2704–2716.
- Koh, T.; DiPietro, L. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23.
- Miao, X.; Leng, X.; Zhang, Q. The current state of nanoparticle-induced macrophage polarization and reprogramming research. Int. J. Mol. Sci. 2017, 18, 336.
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994.
- Jain, S.; Tran, T.; Amiji, M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials 2015, 61, 162–177.
- Corsi, F.; Caputo, F.; Traversa, E.; Ghibelli, L. Not only redox: The multifaceted activity of cerium oxide nanoparticles in cancer prevention and therapy. Front. Oncol. 2018, 8, 309.
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.; Marei, H. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655.
- Choi, J.; Lee, S.; Christ, G.; Atala, A.; Yoo, J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906.
- Mehanna, M.M.; Mohyeldin, S.M.; Elgindy, N.A. Respirable nanocarriers as a promising strategy for antitubercular drug delivery. J. Control. Release 2014, 187, 183–197.
- Caso, M.; Carotenuto, F.; Di Nardo, P.; Migliore, A.; Aguilera, A.; Lopez, C.; Venanzi, M.; Cavalieri, F.; Rinaldi, A. Nanoporous microsponge particles (NMP) of polysaccharides as universal carriers for biomolecules delivery. Nanomaterials 2020, 10, 1075.
- Firouzi-Amandi, A.; Dadashpour, M.; Nouri, M.; Zarghami, N.; Serati-Nouri, H.; Jafari-Gharabaghlou, D.; Karzar, B.; Mellatyar, H.; Aghebati-Maleki, L.; Babaloo, Z.; et al. Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: Possible application in tissue regeneration. Biomed. Pharmacother. 2018, 105, 773–780.
- Wynn, T.; Vannella, K. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462.
- Bartneck, M.; Ritz, T.; Keul, H.; Wambach, M.; Bornemann, J.; Gbureck, U.; Ehling, J.; Lammers, T.; Heymann, F.; Gassler, N.; et al. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano 2012, 6, 8767–8777.
- Wang, L.; Zhang, H.; Sun, L.; Gao, W.; Xiong, Y.; Ma, A.; Liu, X.; Shen, L.; Li, Q.; Yang, H. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnol. 2020, 18, 38.
- Raimondo, T.; Mooney, D. Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10648–10653.
- Shen, X.; Yu, Y.; Ma, P.; Luo, Z.; Hu, Y.; Li, M.; He, Y.; Zhang, Y.; Peng, Z.; Song, G.; et al. Titania nanotubes promote osteogenesis via mediating crosstalk between macrophages and MSCs under oxidative stress. Colloid Surf. B 2019, 180, 39–48.
- Lee, C.; Kim, Y.; Jang, J.; Park, J. Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces. Nanotechnology 2016, 27, 085101.
- Xu, W.C.; Dong, X.; Ding, J.L.; Liu, J.C.; Xu, J.J.; Tang, Y.H.; Yi, Y.P.; Lu, C.; Yang, W.; Yang, J.S.; et al. Nanotubular TiO2 regulates macrophage M2 polarization and increases macrophage secretion of VEGF to accelerate endothelialization via the ERK1/2 and PI3K/AKT pathways. Int J. Nanomed. 2019, 14, 441–455.
- Li, K.; Shen, Q.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Incorporation of cerium oxide into hydroxyapatite coating regulates osteogenic activity of mesenchymal stem cell and macrophage polarization. J. Biomater. Appl. 2016, 31, 1062–1076.
- Li, J.; Wen, J.; Li, B.; Li, W.; Qiao, W.; Shen, J.; Jin, W.; Jiang, X.; Yeung, K.; Chu, P. Valence state manipulation of cerium oxide nanoparticles on a titanium surface for modulating cell fate and bone formation. Adv. Sci. 2017, 5, 1700678.
- Coletti, D.; Scaramuzzo, F.; Montemiglio, L.; Pristerà, A.; Teodori, L.; Adamo, S.; Barteri, M. Culture of skeletal muscle cells in unprecedented proximity to a gold surface. J. Biomed. Mater. Res. A 2009, 91, 370–377.
- Ko, W.; Kim, S.; Heo, D.; Han, I.; Kim, S.; Kwon, I.; Sohn, S. Double layers of gold nanoparticles immobilized titanium implants improve the osseointegration in rabbit models. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102129.
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132.
- Liu, M.; Li, N.; He, Y.; Ge, Y.; Song, G. Dually emitting gold-silver nanoclusters as viable ratiometric fluorescent probes for cysteine and arginine. Microchim. Acta 2018, 185, 147.
- Ishizaki, K.; Sugita, Y.; Iwasa, F.; Minamikawa, H.; Ueno, T.; Yamada, M.; Suzuki, T.; Ogawa, T. Nanometer-thin TiO2 enhances skeletal muscle cell phenotype and behavior. Int. J. Nanomed. 2011, 2011, 2191–2203.
- Lucarelli, M.; Gatti, A.M.; Savarino, G.; Quattroni, P.; Martinelli, L.; Monari, E.; Boraschi, D. Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. Eur. Cytokine Netw. 2004, 15, 339–346.
- Caputo, F.; Giovanetti, A.; Corsi, F.; Maresca, V.; Briganti, S.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium oxide nanoparticles re-establish cell integrity checkpoints and apoptosis competence in irradiated HaCaT cells via novel redox-independent activity. Front. Pharmacol. 2018, 9, 1183.
- Chigurupati, S.; Mughal, M.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials 2013, 34, 2194–2201.
- Siposova, K.; Huntosova, V.; Shlapa, Y.; Lenkavska, L.; Macajova, M.; Belous, A.; Musatov, A. Advances in the study of cerium oxide nanoparticles: New insights into antiamyloidogenic activity. ACS Appl. Bio Mater. 2019, 2, 1884–1896.
- Walczynska, M.; Jakubowski, W.; Wasiak, T.; Kadziola, K.; Bartoszek, N.; Kotarba, S.; Siatkowska, M.; Komorowski, P.; Walkowiak, B. Toxicity of silver nanoparticles, multiwalled carbon nanotubes, and dendrimers assessed with multicellular organism Caenorhabditis elegans. Toxicol. Mech. Methods 2018, 28, 432–439.

