The application of Health Information Technologies (HITs) can be an effective way to advance medical research and health services provision. The two-fold objective of this work is: i) to identify and review the state-of-the-art HITs that facilitate the aims of evidence-based medicine and ii) to propose a methodology for HIT assessment.
The systematic review was conducted according to the PRISMA guidelines. Furthermore, we consolidated existing knowledge in the field and proposed a Synthesis Framework for the Assessment of Health Information Technology (SF/HIT) in order to evaluate the joint use of Randomized Controlled Trials (RCTs) along with HITs in the field of evidence-based medicine.
55 articles met the inclusion criteria and refer to 51 (RCTs) published between 2008 and 2016. Significant improvements in healthcare through the use of HITs were observed in the findings of 31 out of 51 trials - 60.8%. We also confirmed that RCTs are valuable tools for assessing the effectiveness, acceptability, safety, privacy, appropriateness, satisfaction, performance, usefulness and adherence.
To improve health service delivery, RCTs apply and exhibit formalization by providing measurable outputs. Towards this direction, we propose the SF/HIT as a framework which may help the researchers to carry out the appropriate evaluations and extend their studies.
- Health Information Technology
- Evidence-based medicine
- Evidence-based Health Informatics
- Healthcare Quality
- Systematic Review
IAccording to the key role of information technology in every aspect of health care, the aforementioned benefits have to be validated. Thus, we identified four broad Research Questions (RQs) t[1]hat will guide the rest of our work:
- RQ1. Are there any classification methods for the detailed description of the results associated with HITs and to what extend? It remains unclear whether the advances of HITs in recent years take into account or suggest such evaluation models or standards.
- RQ2. Is there any framework or methodology to follow for the assessment of HITs in order to evaluate, compare or extend results from other studies and systematic reviews? It is uncertain whether comprehensive and standardized assessment frameworks / methodologies have been proposed or if further investigation and study is required in the field.
- RQ3. Which are the most established HITs (i.e. HIT forms in accordance with their functional capabilities and the categories of applied Information science) that support evidence-based medicine and are integrated in medical and nursing practices?
- RQ4. What are the features, the outcomes and the types of HIT interventions of the included studies in this research, and their classification in accordance with the medical/health domain?
n this work (addressing RQ1 and RQ3), we aim at identifying the functionalities t[2]hat can be used to prototype health care delivery and to promote health IT evaluation.
With respect to RQ2, we consolidated the aforementioned existing knowledge and propose a Synthesis Framework for the Assessment of Health Information Technology (SF/HIT) for evidence-based medicine that employs HITs (Figure 1). SF/HIT consists of two main categories: a) the Health Core Domains category (Table 1 in supplementary file sup_basic.pdf) and b) the Health Information Technology category . SF/HIT aims at filling the gap of HITs assessment in evidence based medicine and towards this goal it encompasses both the technological aspect of HITs and the outcomes evaluation from the health domain perspective.
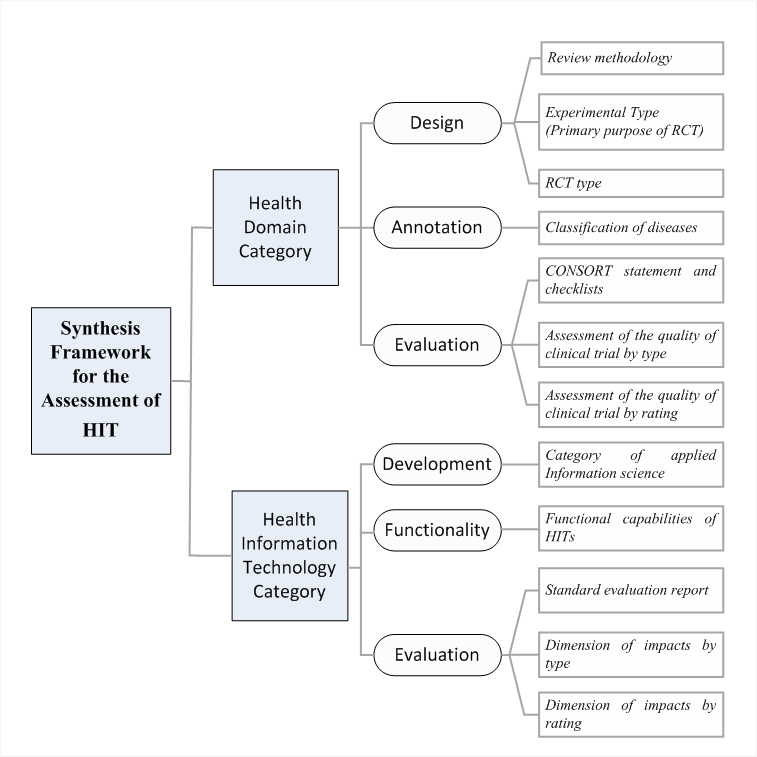
Figure 1: Synthesis Framework for HTΑ
According to this methodology, the Health Core Domains category includes the findings associated with health care provision, and is divided in three sub-categories, namely a) design, b) annotation and c) evaluation.
In the design sub-category we consider i) the PRISMA 2009 methodological tool , ii) the taxonomy related with the experimental type (e.g. prevention, screening, treatment, etc.) of the RCTs as well as iii) the taxonomy related with the RCT type e.g. open, single blind, etc.). In the annotation sub-category, the trials are annotated under the ICD-10 medical terminology. Finally, in the evaluation sub-category we consider i) the CONSORT statement and checklist , ii) the assessment of the quality of clinical trial by type and iii) the assessment of the quality of clinical trial by rating. The first comprises a 25-item checklist and a flow diagram to provide additional guidance for RCTs, while the second and third are used in order to evaluate bias type (e.g. selection bias, performance bias, attrition bias etc.) and its level (e.g. high, low, undefined) respectively.
The Health Information Technology category includes the findings associated with the technical aspect of the interventions. Similarly, this category contains three sub-categories, namely a) development, b) functionality and c) evaluation.
The development sub-category, uses a taxonomy based on the Medical Subject Headings (MeSH) classification system (Figure 2).
The functionality sub-category, consists of 5 HITs types CBA (Computer based alerts and reminders systems), CPOE (Computerized Physician Order Entry), DSS (Decision Support Systems), EHR (Electronic Health Record) and other type) that describe the functional capabilities of HITs.
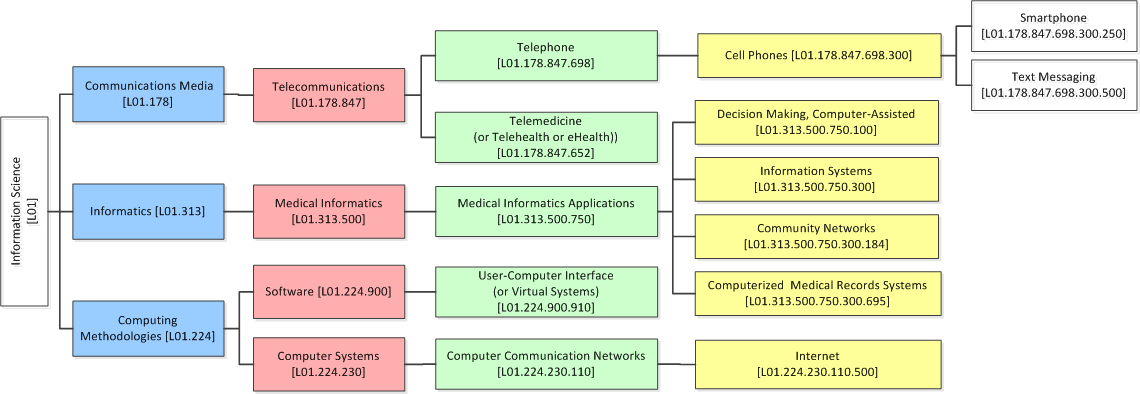
Figure 2: Taxonomy of HITs based on MeSH terminology
Finally, the evaluation sub-category, includes i) the CONSORT-EHEALTH Checklists ii) the impacts by type, and iii) the impacts by rating. The CONSORT-EHEALTH Checklists stands as a standard evaluation report, while the impacts in the reviewed studies (e.g. in the preventive care, adherence/attendance, efficiency, usefulness, effectiveness etc.) are recorded.
References
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. G Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine 2009, 151 (4), 264--269, 10.1371/journal.pmed.1000097.
- Lipscomb Medical subject headings (MeSH). Medical subject headings (MeSH) 2000, 88 (3), 265-6, --.
