- heat-shock proteins
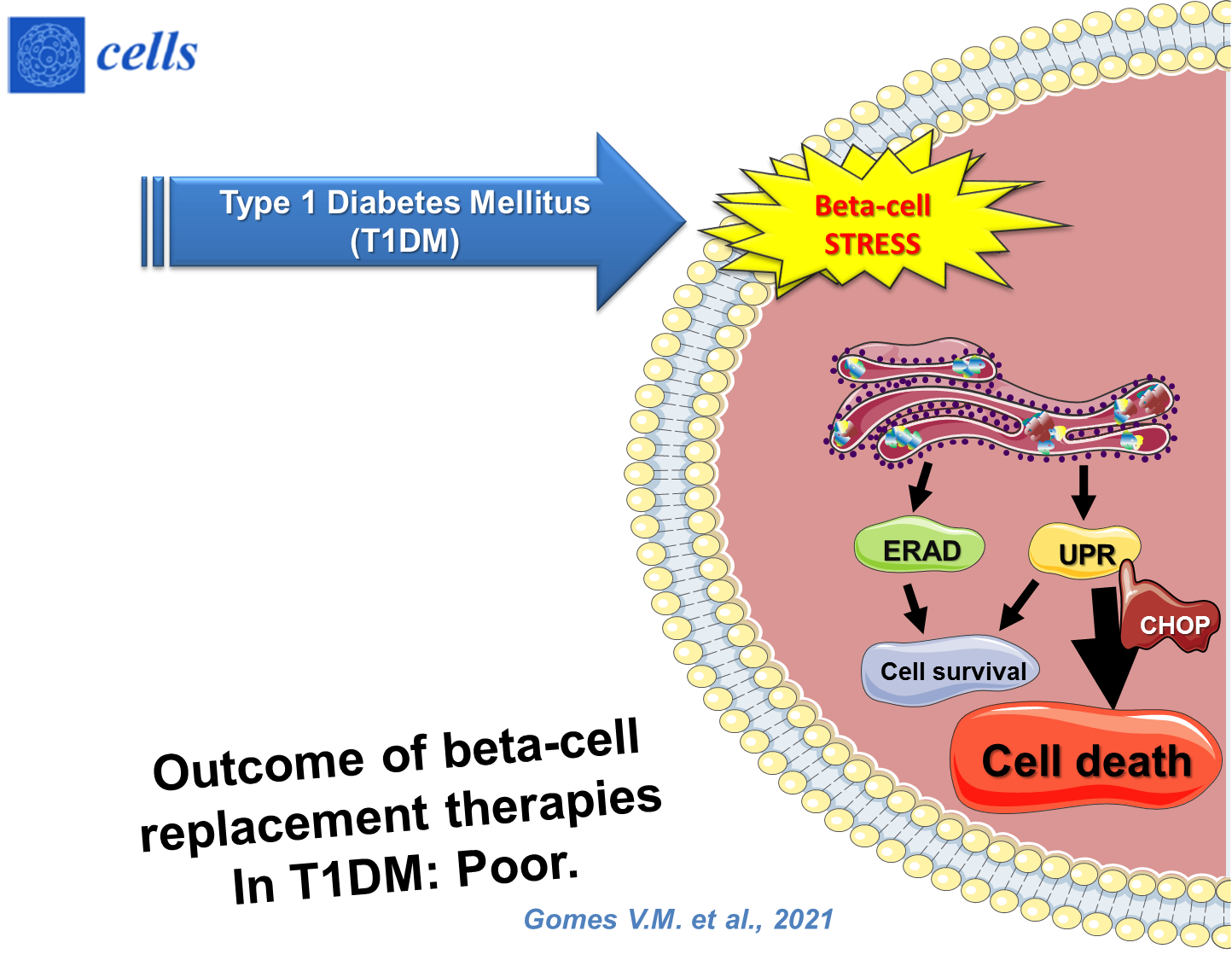
- diabetes mellitus
- beta-cells
- endoplasmic reticulum stress
- HSPB1
- beta-cell transplantation
- prolactin
During type 1 diabetes mellitus (T1DM) development, beta-cells undergo intense endoplasmic reticulum (ER) stress that could result in apoptosis through the failure of adaptation to the unfolded protein response (UPR). Islet transplantation is considered an attractive alternative among beta-cell replacement therapies for T1DM. To avoid the loss of beta-cells that will jeopardize the transplant’s outcome, several strategies are being studied.
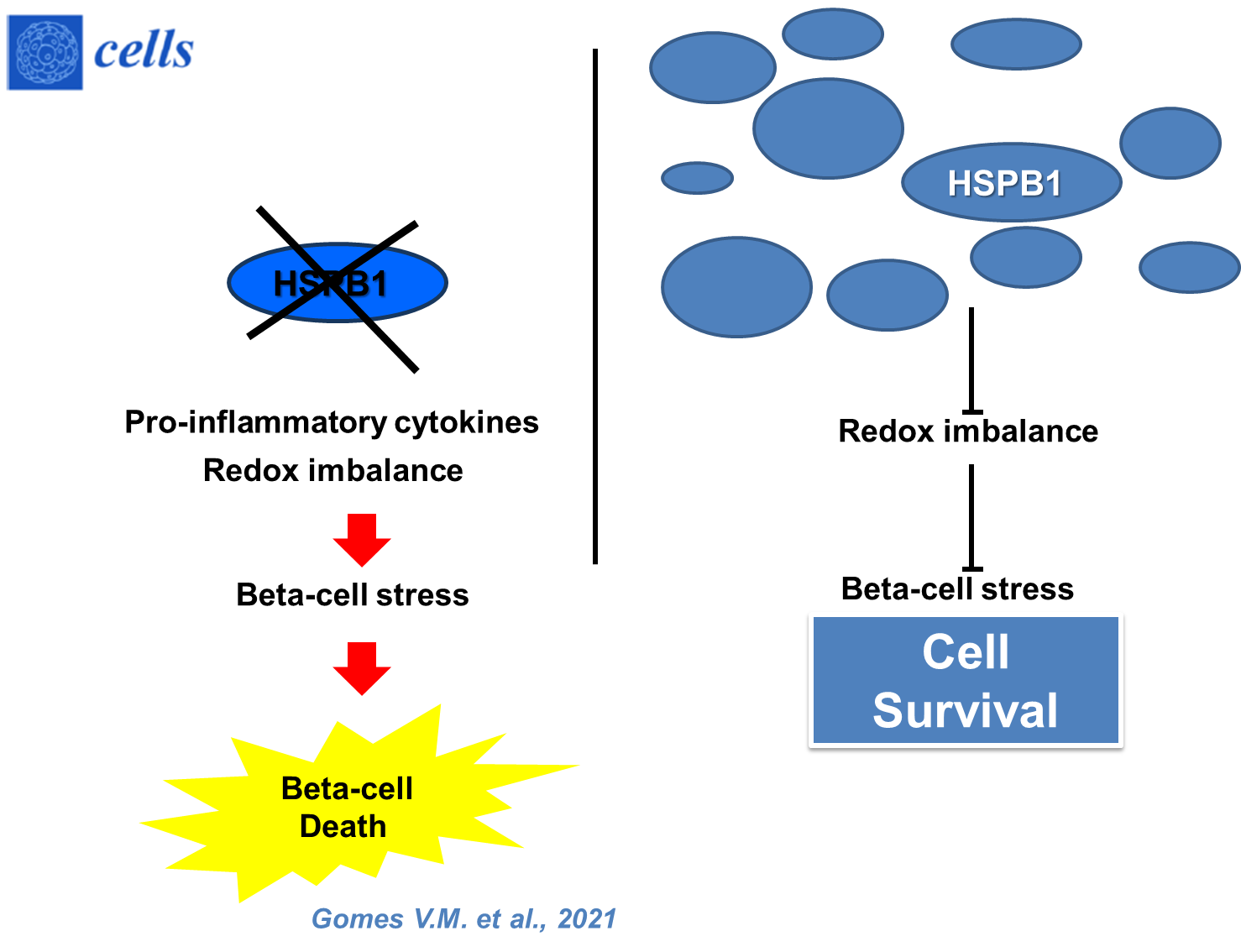
Authors have previously shown that prolactin induces protection against pro-inflammatory cytokines and redox imbalance-induced beta-cell death by increasing heat shock protein B1 (HSPB1) levels.
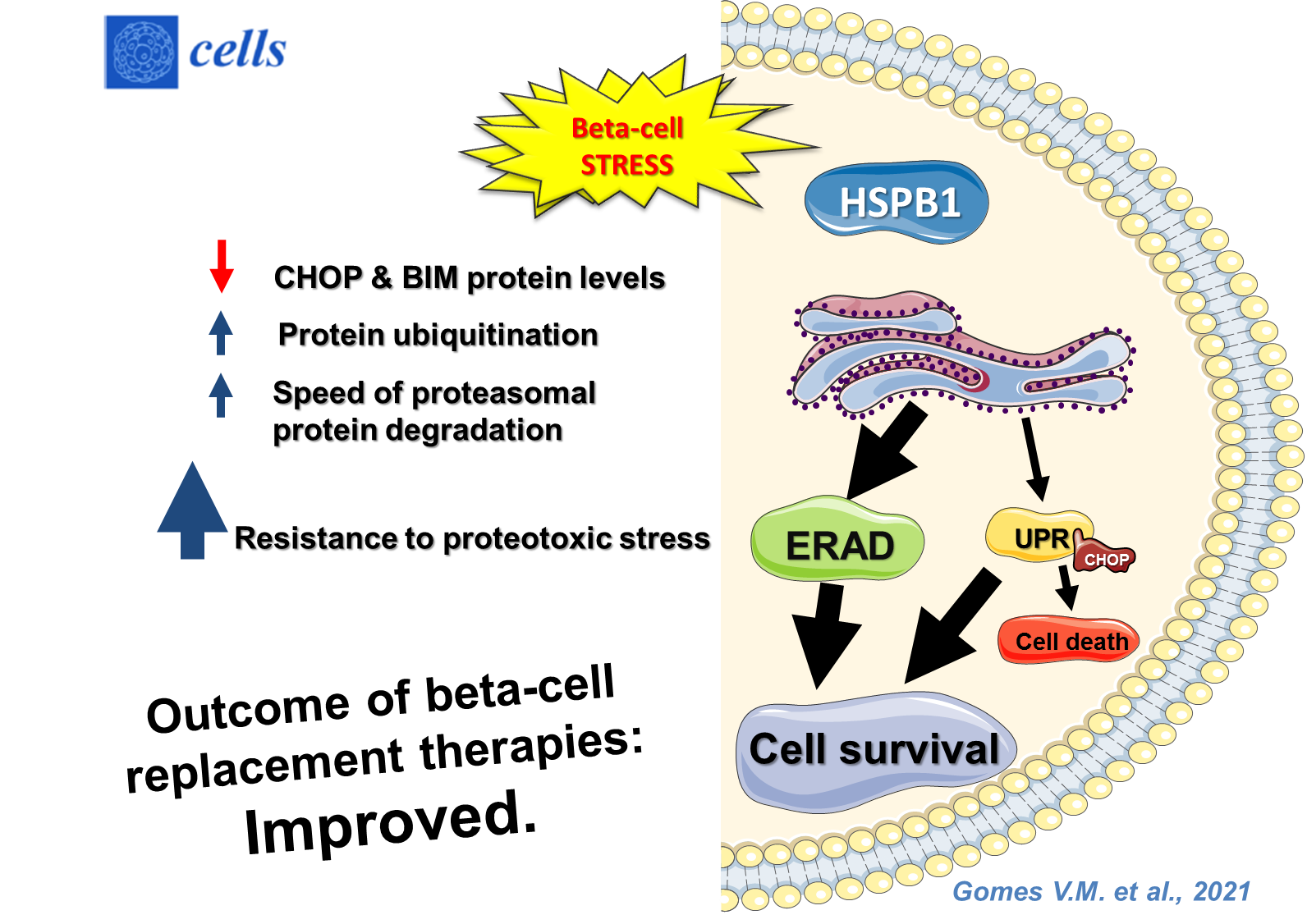
Since the role of HSPB1 in beta cells has not been deeply studied, we investigated the mechanisms involved in unbalanced protein homeostasis caused by intense ER stress and overload of the proteasomal protein degradation pathway. Authors tested whether HSPB1-mediated cytoprotective effects involved UPR modulation and improvement of protein degradation via the ubiquitin-proteasome system.
They demonstrated that increased levels of HSPB1: attenuated levels of pro-apoptotic proteins like CHOP and BIM, increased protein ubiquitination and the speed of proteasomal protein degradation. Their data showed that HSPB1 induced resistance to proteotoxic stress and thus enhanced cell survival via an increase in beta-cell proteolytic capacity. These results could contribute to generate strategies aiming at optimization of beta-cell replacement therapies.