Your browser does not fully support modern features. Please upgrade for a smoother experience.
Subjects:
Cell Biology
1. Introduction
The continuous and increasing usage of fossil fuels has led to their depletion. Moreover, it has introduced various environmental hazards in the form of pollutants [1]. Bioenergy has been declared by the International Energy Agency (IEA) as the highest source of growth, at ~30% in the renewable consumption sector over the period of 2018–2023 [2]. In the year 2017, solar, hydropower, wind, and bioenergy covered ~25% of the power demand [3] around the world. In spite of having multiple renewable energy sources, consistent energy production and even supply are not ensured, because of climate dependency [4]. In this context, biomass degradation and their conversion in to biofuels seems an impressive and promising option, as cellulosic biomass is abundantly available and it is renewable as well [5,6,7,8]. Lignocellulosic biomasses are present in most of a plant’s cell walls, and hence it marks its presence in a plentiful amount [4]. Examples of such lignocellulosic biomasses are waste materials like forest residues, and agricultural, municipal, and industrial activities. Additionally, they can also be grown as energy crops that do not compete with food crops [9,10]. In general, the lignocellulosic biomass is comprised of two polysaccharides—cellulose, a homo-polysaccharide, and hemicelluloses, a hetero-polysaccharide. In addition, an aromatic hydrocarbon lignin is also present, along with a smaller portion of ash, proteins, and pectin [4]. Cellulose is a high molecular weight linear polymer composed of β-glucose (5000–10,000 units) and linked by β-1,4-glycosidic bonds [11]. Moreover, cellulose is highly crystalline in nature, and that makes it challenging to convert into monomeric sugars through the hydrolysis process [12]. Hemicellulose contains approximately 150 repeating monosaccharide (C5 and C6 sugar) units, and the type of monomeric sugars present therein varies depending on the types of materials [11]. In general, before the hydrolysis of a biomass, pretreatment is required so as to break the lignin present in the outermost layer of the biomass. The pretreatment of lignocellulosic biomasses can be done by following physical, chemical, and biological methods using chemicals or enzymes. Nevertheless, these treatment methods may result in the partial or complete removal of the lignin, and release the free structure of cellulose and hemicellulose, which can be further converted in to sugars by enzymes, and subsequently in to fuels, following fermentation using various types of microorganisms [13].
The physical methods used for the pretreatment of the lignocellulosic biomass mainly perform the structural disruption of the lignocelluloses, but require a high energy input such as extrusion [14,15] or cavitation [16,17]. On the other hand, chemical methods are most commonly used for the pretreatment of lignocellulosic biomass through alkali and acidic treatment [18,19]. Although the chemical method is effective, the high cost of the chemical consumption makes this method non-economical for a pilot scale, whereas biological pretreatment via microorganisms is sustainable and environment friendly. After lignin removal through the pretreatment process, the enzymatic bioconversion of free cellulose and hemicellulose yields reducing sugars. The enzymatic hydrolysis of the pretreated biomass requires the cellulolytic enzymes to break the polymeric structure of the cellulose and hemicellulose. Although multiple enzymes such as cellulase, hemicellulase, xylanase, ligninase, and pectinase actively participate in the enzymatic conversion of biomass, cellulase is the most important enzyme, because of its efficiency to perform the complete hydrolysis of cellulose into sugar [20]. Furthermore, cellulase is a system of three different enzymes, namely, exoglucanase, endoglucanase, and beta-glucosidase (BGL), and these enzymes act synergistically for the hydrolysis of cellulose [21,22]. All of these three enzymes perform distinct functions in the complete hydrolysis of cellulose. Endoglucanases randomly act over the crystalline structure of cellulose and cleave the linear chains of glucose, which results in shorter chains giving two new chain ends [23]. These two exposed ends then become available for the action of exoglucanases, which extricate cellobiose and some glucose [23]. Ultimately, the final role is played by β-glucosidases (BGL) for the complete degradation of cellulose, which breaks cellobiose and cello-oligosaccharides into glucose molecules [24,25,26,27]. Thereafter, β-glucosidase is the final enzyme in lignocelluloses degradation, which decides the rate of the total conversion of lignocelluloses into glucose [20,28,29,30,31,32].
As a result of the significant role of β-glucosidase in the biofuel industry, its production is needed so as to enhance and match up with the current demand of industries. For the production of cellulases, Trichoderma reeseihas been widely used, but the yield of β-glucosidases from it is very poor [33]. It has been reported that most of the β-glucosidases produced from T. reesei were found to attach to the cell wall during fungal growth, causing the secretion of β-glucosidase of a low quantity into the medium [34,35]. Hence, the extraction of the β-glucosidase becomes a tedious task, and therefore the production of β-glucosidases is insufficient [34]. Although both the bacterial and fungal microbial strains are well reported for BGL production, the genus Aspergillus of fungi such as Aspergillus niger and Aspergillus Phoenicus are reported to give a higher yield of the β-glucosidases enzyme [36]. The earlier existing commercial β-glucosidase enzyme Novozyme SP188 was produced from A. niger, on which higher concentrations of glucose showed deleterious effects [37]. Sorensen et al. in their study described a novel species, Aspergillus saccharolyticus, that was able to produce an even higher titer value of β-glucosidases than A. niger, and can also substitute the commercial production of BGL [38]. In addition, some bacterial strains have been reported as potential β-glucosidase producers, such as Bacillus subtilis [39] and Acidothermus cellulolyticus [40], which produce more thermostable BGL compared with fungi, but are slow producers. In a recent study, Chen et al. described the cloning of the BGL gene from Bacillus licheniformis into Escherichia coli, and the production of the β-glucosidase enzyme with45.44 U/mLactivity [41]. Furthermore, it is also well documented that the activity of β-glucosidases gets inhibited because of the feedback inhibition of the final product glucose [20]. This loophole has led to an increased number of findings about glucose tolerant and glucose stimulated β-glucosidases, dating back the last 20 years [42]. In a study, Tiwari et al. worked on B. subtilis RA10 for the production of the thermostable β-glucosidase, which could efficiently convert cellulosic biomass into fermentable sugar [43]. Researchers also tried to increase the β-glucosidases production using various strategies. The enhanced production of β-glucosidases may be achieved by co-culturing Trichodermareesei with some other fungi, resulting in the proliferation of the enzyme efficiency of cellulose hydrolysis [44,45]. At the same time, recombinant DNA technology offers an alluring option for the cost cutting of the process by developing recombinant T. reesei, which can produce an absolute saccharifying enzyme in an ideal amount, containing β-glucosidases [46]. In addition, the β-glucosidase activity may be increased by the heterologous expression of β-glucosidase from other fungi in T.reesei, such as Neosartorya fischeri [47], Aspergillus aculeatus [48,49], and Periconia sp. [50]. Still, no recombinant strain of T. reesei is available that could ideally produce all of the components of cellulase, regardless of insight, knowledge, and multiple genetic efforts.
In view of the above facts, the present review evaluates the production and efficiency of the β-glucosidase enzyme in the bioconversion of the cellulosic biomass for the biofuel production process at an industrial scale. The significance of BGL in biomass conversion has been discussed along with the recent developments and existing issues. The production and development of the microbial BGL enzyme have been also explained in detail, along with the recent advancements made in this field. At last, the current hurdles and future suggestions have been provided for the further developments.
2. Industrial Importance of β-Glucosidase in Biofuels
Beta-glucosidase is a dual character enzyme that incorporates both the synthesis and degradation of the glycosidic bond, and this attribute of β-glucosidase makes it an enzyme with enormous potential from an industrial point of view [51,52]. The current scenario of raising the global energy demand day by day, and the increasing burden on fossil fuels, have necessitated biofuels production at a large scale so as to replace fossil fuels. The process of cellulosic biofuel production includes the breakdown of lignocellulosic biomass into sugar, followed by biofuel production through the fermentation process [20,52,53]. BGL is the key enzyme that ultimately converts cellobiose and cellooligosaccharide into a monomeric unit of glucose [54,55,56]. However, because of the insufficient BGL production, it becomes a rate limiting step of biofuel production technology [57,58]. Figure 1represents the production process of the BGL enzyme at an industrial scale, using the fungal microorganism A. niger [59].
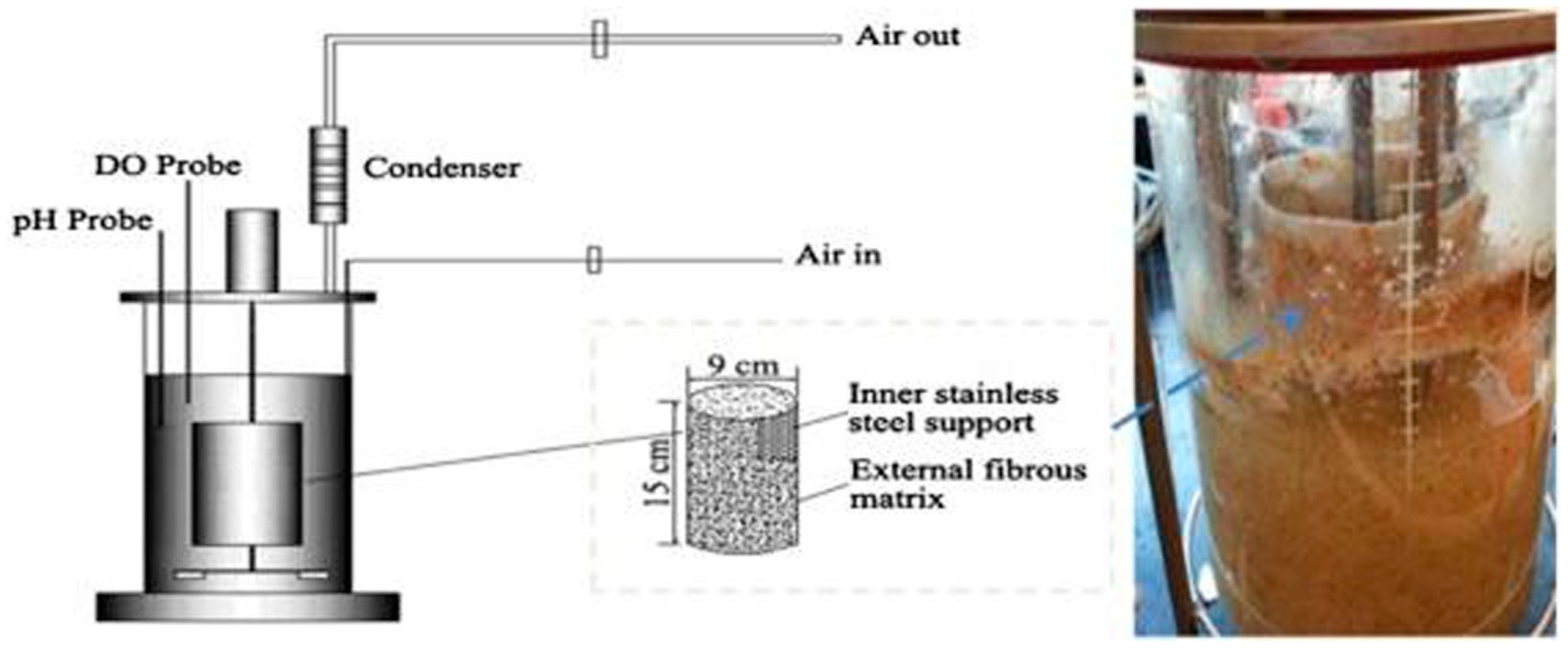
Figure 1. Higher β-1,4-glucosidase production by Aspergillus niger grown on wheat bran and glycerol was obtained in a rotating fibrous bed bioreactor (RFBB), because of better morphology control and mass transfer (adopted with permission from the authors of [59]).
It has been noticed that because of the low efficiency of β-glucosidase and the unavailability of the potential BGL producer microorganism, the total conversion rate of cellulose to sugar is usually low. Moreover, the accumulation of cellobiose inhibits the other two enzymes of the cellulase complex, exoglucanase and endoglucanase [60,61]. The rate of hydrolysis, inhibitors, and stability, along with the product inhibition and thermal instability, are some of the major key factors that are needed in order to focus on achieving a higher conversion rate using BGL [58]. For example, a slow rate of hydrolysis and product inhibition have been noticed as the rate limiting step [62,63], while these bottlenecks can be overcome well by use the of [64,65] thermostable β-glucosidase, which has been documented in several studies [66,67]. Moreover, various research has been also performed, primarily focused on the heterologous expression and enzymatic cocktails, resulting in novel enzyme mixtures [68,69]. In a study, Chen et al. [70] worked on Pichia pastoris, which was expressing β-glucosidase encoding cDNA segregated from Neocallimastix patriciarum, a buffalo rumen fungus. This engineered enzyme showed better saccharification than the commercially available Novozym 188. In another study, Lee et al. modified Saccharomyces cerevisiae by the expression of the β-glucosidase and cellodextrin transporter from Neurospora crassa, and concluded the concomitant saccharification and fermentation that leads to a cost reduction [71]. In a recent work on the recombinant E. coli, Ferreira et al. [72] designed a new model for the cost effective production of β-glucosidase, and noticed the BGL had a yield of 15 g/L, making the final production cost ~37 US$/kg.
For achieving the goal of economic biofuel production, many researchers have been working on different fungal and bacterial strains so as to obtain an effective cellulolytic fungal or bacterial β-glucosidase [73]. Liu et al. [74] reported on the increased hydrolysis of sugarcane bagasse with the help of BGL produced from Anoxybacillus flavithermus subsp.yunnanensis E13T. Yan et al., in their study, performed the hydrolysis of soybean isoflavones through the BGL obtained from Aspergillus terreus [75]. Liu et al. worked on Aspergillus fumigatus Z5, which gave a thermostable β-glucosidase, which was active even at an elevated temperature, and also, when added to the lignocellulosic biomass, it resulted in the removal of phenolic compounds, and hence it may be used for the degradation of polyphenols [76].
The use of thermostable and thermophilic β-glucosidase in cellulose hydrolysis is of particular interest, as a higher temperature of the process would not hinder the activity of BGL, and it also favors the hydrolysis process of the cellulose [77]. Multiple studies are present that describe the thermophilic and thermostable β-glucosidase. Dashtban and Qin isolated the thermostable BGL gene from Periconia sp., and inserted it into T. reesei, and hence the produced BGL showed an optimum activity at 60 °C [50]. Another study by Tiwari et al. involved Bacillus subtilisRA10 that produced thermostable BGL with 78% activity at 80 °C [43]. Zhang et al. isolated a thermophilic β-glcosidase from Thermotoga naphthophila RUK-10 and used it with cellulase for the hydrolysis of untreated corn straw, and observed an increase of 93.5% in the conversion rate from cellulose to glucose [77]. In a recent study, Fusco et al. synthetically produced a gene Dtur_0462 coding or β-glucosidase from Dictyoglomus turgidum and expressed in a strain of Escherichia coli, which resulted in the production of a thermostable β-glucosidase DturβGlu. The maximum activity of the produced β-glucosidase was observed at 80 °C, and it was able to exhibit 70% activity after the incubation of 2 h at 70 °C [78]. It was also described by Yeoman et al. that Sclerotium glucanicum [79] and Aspergillus pheonicis [80] mesophilic fungi grown at 24–27 °C produces β-glucosidase with an optimum temperature and stability of about 60–75 °C, and hence these fungi may be useful in order to produce thermophilic BGL at a normal temperature [81].
The co-culturing of different fungi for a higher yield of β-glucosidase has also been the focus of researchers [82,83,84,85]. Hu et. al. worked with A. niger and Aspergillus oryzae, along with some other strains like Magnaporthe grisea or Phanerochaete chrysosporium. The co-culturing of these strains displayed an improved production of enzymes, and the highest β-glucosidase activity was found for A. oryzae with P. chrysosporium [86]. Trichoderma virdiae, which produces the most commonly used cellulase enzyme, has a measurable β-glucosidase activity, and therefore the incorporation of thermo-tolerant BGL into cellulase preparation may give value addition effects, and hence an increased sugar concentration [31]. In a recent study, Zhao et al. combined the benefits of co-culturing and genetic modification by using the recombinant T. reesei mixed with the A. niger culture. This approach resulted in the most powerful cellulase with the highest enzymatic hydrolysis giving a yield of 89.35%, and for 1g of glucose production it needed the lowest input of cellulase (i.e., 25.18 filter paper unit FPU) [85]. Mallerman et al. worked on Flammulina velutipes CFK 3111, a white rot fungus, and observed the maximal β-glucosidase production of 1.6 U/mLwith a glucose production of ~10 g/L [87]. In a study, Jongmin et al. isolated Aspergillus sp.YDJ216, which produced two β-glucosidases, BGL1 and BGL2, and out of these two, BGL1 exhibited the maximum activity (953.2 U/mg) [88]. Abdella et al. worked on A. niger, and found the maximum BGL production at the repeated batch mode in the rotating fibrous bed bioreactor (RFBB) of about 1.78 U/mL/day [59]. In all of the above-mentioned studies on the BGL production, the best result was observed from the co-culturing of the recombinant T. reesei with A. niger, which has the potential of being used as a commercial producer of β-glucosidase [85].
Apart from biofuel production, β-glucosidase plays an important role in the beverage industry [24,32,57,89,90]. In wine production, BGL helps in the removal of the aromatic compound from the precursors of the glucosides present in fruit juices and musts [32,57], in the flavoring of tea [91], and fruit juice [90]. While working on the flavor and aroma enhancement of white muscat wine, Gonzalez-Pombo et al. [92] used Issatchenkia terricola for the isolation of β-glucosidase, which improved the flavor of the wine. Apart from beverages, the role of β-glucosidase is very fascinating in foods, especially those made from soy [93]. Glycosidic isoflavones, which are present in soy, are mainly daidzin, genistin, and glycitin [52]. These are largely inactive glycosides and need the activity of β-glucosidase to get them to convert into aglycones, namely daidzein, genistein, and glycitein [93]. Fermentation with a lactobacillus, producing β-glucosidase, or by treatment with β-glucosidase, resulted in a significant increase of aglycon in soy milk [94]. In addition, BGL synthesized a number of β-glucosides, abioflavanoid molecules that bond to glucose and are considered a subtype of glycosidase in plants. Additionally, because of its involvement in fundamental biological processes, multiple reports on its potential applications have been documented by the researchers, such as the hydrolysis of glucosyl ceramides in mammals and humans [95,96], the formation of glycoconjugates that play role in the defense mechanism of plants and microbes [24]. Beta-glucosidase acts on the precursors of glucosides found in fruits, and helps in the removal of aromatic compounds [97]. Because of this property, BGL is a crucial enzyme in the flavor industry. By performing reverse hydrolysis, β-glucosidase may also be used to synthesize the surfactant o-alkyl-glucoside, which may perform biological degradation, and also in food industry as a detergent [33]. Waste paper is currently a major environmental pollutant, and recycling it will give two-fold benefits, by reducing the consumption of forest wood and reducing landfill pollution [53]. There are two methods for recycling paper waste, either by the chemical or the enzymatic method, and because chemicals are environmentally hazardous, the use of enzymes like cellulase, β-glucsoidase, and hemicellulase is recommended [98,99,100,101]. Table 1 summarizes the numerous studies on the production of the BGL enzyme using the microbial process, following different physiological conditions, with the potential for biofuel application.
Table 1. Various microorganisms producing β-glucosidases under different physiological conditions and the activity of produced beta-glucosidase (BGL) in (IU/mL).
| S.No | Microorganism | Physiological conditions(Temperature, pH, Mode of fermentation and substrate) | BGL Activity | Ref. | |||
|---|---|---|---|---|---|---|---|
| Temp | pH | Mode of Fermentation | Substrate | (IU/mL) | |||
| 1. | Trichodermaatroviridae TUB F-1505 TUB F-1724 TUB F- 1753 |
30 °C 30 °C 30 °C |
6.2 6.2 6.2 |
Submerged Submerged Submerged |
Steam pretreated willow | 5.30 11.70 10.28 |
[102] |
| 2. | Bacillus halodurans C-125 | 45 °C | 8.0 | Submerged | Lactose induced Luria broth LB media | 95 | [103] |
| 3. | Aspergillusprotuberus | 30 °C | 3.0 | Solid state | Rice husk | 26.06 IU/g | [104] |
| 4. | Pichiapastoris Bgl gene from Aspergillus niger |
30 °C | 5.0 | Fed batch | Glycerol+ methanol(1:5 ratio) | 129 | [105] |
| 5. | Candida peltata NRRL Y-6888 | 50 °C | 5.0 | Submerged | Glucose+xylose+sucrose+ maltose+arabinose | 1.5 | [106] |
| 6. | Issatchenkiaorientalis | 50 °C | 5.0 | Submerged | Esculine | 6 × 10−3 | [107] |
| 7. | Bacillus licheniformis | 60 °C | 7.0 | Submerged | Glucose+ sucrose | 45.44 | [108] |
| 8. | Penicillium oxalicum | 30 °C | - | Submerged | Microcrystalline cellulose | 150 | [109] |
| 9. | Talaromycesamestolkial | 70 °C | 4.0 | Submerged | Glucose | 1.8 | [110] |
| 10. | Penicillium piceum | 55 °C | 5.0 | Submerged | Avicel | 53.12 | [111] |
| 11. | Penicilliume chinulatum | 50 °C | 4.8 | Submerged | Microcrystalline cellulose+glucose+ soy bran | 1.5 | [111] |
| 12. | Saccharophagus degradans, 2-40T | 30 °C | 6.0 | Submerged | Laminarin | - | [112] |
| 13. | Micrococcus antarcticus | 25 °C | 6.5 | Submerged | Cellobiose | 289 | [113] |
| 14. | Aspergillus awamori | 28 °C | 4.5 | Solid state | Pineapple crown leaves + wheat bran | 820 ± 30 IU/g | [114] |
| 15. | Aspergillus awamori2B.361 U2/1 | 30 °C | 8.0 | Submerged | Wheat bran | 104.7 | [115] |
| 16. | Penicilliumsp. LMI01 | 60 °C | 6.0 | Submerged | Carboxymethyl cellulose | 0.058 ± 0.004 | [116] |
| 17. | Aspergillus niger and Aspergillus oryzae | 28–30 °C | - | Solid state | Sugarcane bagasse | 814 IU/g | [117] |
| 18. | Aspergillus flavus | 37 °C | - | Submerged | Wheat bran | 0.64 | [118] |
| 19. | Aspergillus flavus ITCC 7680 | 30 ± 2 °C | 4.8 | Solid state | Pretreated cotton stalk | 96 ± 2.9 IU/g | [119] |
| 20. | Bacillus subtilisCCMA 0087. | 36.6 °C | 3.64 | Submerged | Coffee pulp | 22.59 | [120] |
| 21. | Lichtheimia ramosa | 32 °C | - | Submerged | Flaxseed | 3.54 | [121] |
References
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33.
- Available online: https://www.iea.org/renewables2018 (accessed on 4 June 2019).
- Available online: https://renewablesnow.com/news/renewables-supply-25-of-global-power-in-2017-iea-606070/ (accessed on 4 June 2019).
- Koupaie, E.H.; Dahadha, S.; Lakeh, A.B.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production—A review. J. Environ. Managem. 2019, 233, 774–784.
- Gaurav, N.; Sivasankari, S.; Kiran, G.S.; Ninawe, A.; Selvin, J. Utilization of bioresources for sustainable biofuels: A Review. Renew. Sustain. Energy Rev. 2017, 73, 205–214.
- Landis, D.A.; Gratton, C.; Jackson, R.D.; Gross, K.L.; Duncan, D.S.; Liang, C.; Meehan, T.D.; Robertson, B.A.; Schmidt, T.M.; Stahlheber, K.A.; et al. Biomass and biofuel crop effects on biodiversity and ecosystem services in the North Central US. Biomass Bioenergy 2018, 114, 18–29.
- Khan, I.U.; Othman, M.H.D.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Azelee, I.W. Biogas as a renewable energy fuel. A review of biogas upgrading, utilization and storage. Energy Convers. Manag. 2017, 150, 277–294.
- Lyytimaki, J. Renewable energy in the news. Environmental, economic policy and technology discussion of biogas. Sustain. Prod. Consum. 2018, 15, 65–73.
- Frigon, J.C.; Mehta, P.; Gelot, S.R. Impact of mechanical, chemical and enzymatic pretreatments on the methane yield from the anaerobic digestion of switch grass. Biomass Energy 2012, 36, 1–11.
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73.
- Takellapati, S.; Li, T.; Gozalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630.
- Brethauer, S.; Studer, M.H. Biochemical conversion process of lignocellulosic biomass to fuel and chemicals—A review. Chim. Int. J. Chem. 2015, 69, 572–581.
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansaw, J.A.; Mohd Falik, A.A.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. 2017, 10, 52–61.
- Hjorth, M.; Gränitz, K.; Adamsen, A.P.; Møller, H.B. Extrusionasapretreatmentto increasebiogasproduction. Bioresour. Technol. 2011, 102, 4989–4994.
- José, M.O.; María, J.N.; Paloma, M.; Ignacio, B.; Miguel, Á.C.; Felicia, S.; Mercedes, B.; Antonio, D.M. A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation 2017, 3, 15.
- Patil, P.N.; Gogate, P.R.; Csoka, L.; DregelyiKiss, A.; Horvath, M. Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ultrason. Sonochem. 2016, 30, 79–86.
- Ruly, T.H.; Lucas, R.; da Silva, S.S.; Giuliano, D.; Solange, I.M.; dos Santos, J.C. Hydrodynamic cavitation as a strategy to enhance the efficiency of lignocellulosic biomass pretreatment. Crit. Rev. Biotechnol. 2018, 38, 483–493.
- Talha, Z.; Ding, W.; Mehryar, E.; Hassan, M.; Bi, J. Alkaline pretreatment of sugarcane bagasse and filtermud co-digested to improve biomethane production. BioMed Res. Int. 2016, 2016, 1–10.
- Shetty, D.J.; Kshirsagar, P.; Tapadia-Maheshwari, S.; Lanjekar, V.; Singh, S.K.; Dhakephalkar, P.K. Alkali pretreatment at ambient temperature: A promising method to enhance biomethanation of rice straw. Bioresour. Technol. 2017, 226, 80–88.
- Reeta, R.S.; Anil, K.P.; Rajeev, K.; Sukuamaran, C.L.; Ashok, P. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour. Tech. 2013, 127, 500–507.
- Satyamurthy, P.; Jain, P.; Balasubramanya, R.H.; Vigneshwaran, N. Preparation and characterization of cellulasenanowhiskers from cotton fibres by controlled microbial hydrolysis. Carbohydr. Polym. 2011, 83, 122–129.
- Binod, P.; Gnansounou, E.; Sindhu, R.; Pandey, A. Enzymes for second generation biofuels: Recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 5, 317–325.
- Zhang, X.-Z.; Zhang, Y.-H.P. Cellulases: Characteristics, Sources, Production, and Applications. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers, 1st ed.; Yang, S.-T., Hesham, A.E.-E., Nuttha, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 131–146.
- Bhatia, Y.; Mishra, S.; Bisaria, V. Microbial b-glucosidases: Cloning, properties, and applications. Crit. Rev. Biotechnol. 2002, 22, 375–407.
- Tiwari, R.; Singh, S.; Shukla, P.; Nain, L. Novel cold temperature active b-glucosidase from Pseudomonas lutea BG8 suitable for simultaneous saccharification and fermentation. RSC Adv. 2014, 4, 58108–58115.
- Cairns, J.R.K.; Esen, A. β-Glucosidase. Cell. Mol. Life Sci. 2010, 67, 3389–3405.
- Li, D.; Li, X.; Dang, W.; Tran, P.L.; Park, S.; Oh, B.; Hong, W.; Lee, J.; Park, K. Characterization and application of an acidophilic and thermostable b-glucosidase from Thermofilumpendens. J. Biosci. Bioeng.2013, 115, 490–496.
- Nigam, D.; Asthana, M.; Kumar, A. Penicillium: A Fungus in the Wine and Beer Industries. In New and Future Developments in Microbial Biotechnology and Bioengineering; Vijai, K.G., Susana, R.-C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 187–200.
- Harhangi, H.R.; Steenbappers, P.J.M.; Akhmanova, A.; Jetten, M.S.M.; Van der Drift, C.; Op den Camp, H.J.M. A highly expressive family 1 β-glucosidase with transglycosylation capacity from the anaerobic fungus Piromyces sp. E2. Biochem. Biophys. Acta 2002, 1574, 293–303.
- Zhang, Y.; Min, Z.; Qin, Y.; Ye, D.-Q.; Song, Y.; Liu, Y.-L. Efficient Display of Aspergillus niger β-glucosidase on Saccharomyces cerevisiae cell wall for aroma enhancement in wine. J. Agric. Food Chem 2019, in press.
- Wilkowska, A.; Pogorzelski, E. Aroma enhancement of cherry juice and wine using exogenous glycosidases from mould, yeast and lactic acid bacteria. Food Chem. 2017, 237, 282–289.
- Keerti, G.A.; Kumar, V.; Dubey, A.; Verma, A.K. Kinetic Characterization nd effect of immobilized thermostable β-glucosidase in alginate gel beads on sugarcane juice. ISRN Biochem. 2014, 2014, 1–8.
- Rani, V.; Mohanram, S.; Tiwari, R.; Nain, L.; Arora, A. Beta-Glucosidase: Key Enzyme in Determining Efficiency of Cellulase and Biomass Hydrolysis. J. Bioproces Biotech. 2014, 5, 197.
- Mark, J.; Dwight, E.T. Mechanism for β-glucosidase release into cellulose-grown Trichoderma reesei culture supernatants. Exp. Mycol. 1988, 12, 203–216.
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Factories 2016, 15.
- Oh, J.M.; Lee, J.P.; Baek, S.C.; Jo, Y.D.; Kim, H. Characterization of Three Extracellular β-Glucosidases Produced by a Fungal Isolate Aspergillus sp. YDJ14 and Their Hydrolyzing Activity for a Flavone Glycoside. J. Microbiol. Biotechnol. 2018, 28, 757–764.
- Marie, C.; Hugues, M.; Delphine, H.; Dominique, C.; Frédéric, M.; Nicolas, L.F. Comparative kinetic analysis of two fungal β-glucosidases. Biotechnol. Biofuels 2010, 3, 1–8.
- Annette, S.; Ahring, B.K.; Lübeck, M.; Ubhayasekera, W.; Bruno, K.S.; Culley, D.E.; Lübeck, P.S. Identifying and characterizing the most significant β-glucosidase of the novel species Aspergillus saccharolyticus. Can. J. Microbiol. 2012, 58, 1035–1046.
- Bagudo, A.I.; Argungu, A.U.; Aliero, A.A.S.; Suleiman, N.; Kalpana, S. Bacillus subtilis as an Alternative Source of Beta-glucosidase. Int. J. Mod. Cell. Mol. Biotechnol. 2014, 3, 1–9.
- Li, Y.; Bu, M.; Chen, P.; Li, X.; Chen, C.; Gao, G.; Feng, Y.; Han, W.; Zhang, Z. Characterization of a Thermophilic Monosaccharide Stimulated β-Glucosidase from Acidothermus cellulolyticus. Chem. Res. Chin. Univ. 2018, 34, 212–220.
- Chen, Z.; Meng, T.; Li, Z.; Liu, P.; Wang, Y.; He, N.; Liang, D. Characterization of a beta-glucosidase from Bacillus licheniformis and its effect on bioflocculant degradation. AMB Express 2017, 7, 197.
- José, C.S.S.; Luana, P.M.; Sibeli, C.; Ward, R.J. Glucose tolerant and glucose stimulated β-glucosidases—A review. Bioresour. Technol. 2018, 267, 704–713.
- Tiwari, R.; Singh, P.K.; Singh, S.; Nain, P.K.S.; Nain, L.; Shukla, P. Bioprospecting of novel thermostable β-glucosidase from Bacillus subtilis RA10 and its application in biomass hydrolysis. Biotechnol. Biofuels 2017, 10, 246–263.
- Treebupachatsakul, T.; Nakazawa, H.; Shinbo, H.; Fujikawa, H.; Nagaiwa, A.; Ochiai, N.; Kawaguchi, T.; Nikaido, N.; Totani, K.; Shioya, K.; et al. Heterologously expressed Aspergillus aculeatus β-glucosidase in Saccharomyces cerevisiae is a cost-effective alternative to commercial supplementation of β-glucosidase in industrial ethanol production using Trichoderma reesei cellulases. Biosci. Bioeng. 2016, 121, 27–35.
- de França Passos, D.; Pereira, N., Jr.; de Castro, A.M. A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Curr. Opin. Green Sustain. Chem. 2018, 14, 60–66.
- Li, C.; Lin, F.; Li, Y.; Wei, W.; Wang, H.; Qin, L.; Zhou, Z.; Li, B.; Wu, F.; Chen, Z. A β-glucosidase hyper production Trichodermareesei mutant reveals a potential role of cel3D in cellulase production. Microb. Cell Fact. 2016, 15, 151–163.
- Xue, X.; Wu, Y.; Qin, X.; Ma, R.; Luo, H.; Su, X.; and Yao, B. Revisiting overexpression of a heterologous β-glucosidase in Trichoderma reesei: Fusion expression of the Neosartorya fischeri Bgl3A to cbh1 enhances the overall as well as individual cellulase activities. Microb. Cell Fact. 2016, 15.
- Nakazawa, H.; Kawai, T.; Ida, N.; Shida, Y.; Kobayashi, Y.; Okada, H.; Tani, S.; Sumitani, J.; Kawaguchi, T.; Morikawa, Y.; et al. Construction of a recombinant Trichodermareesei, strain expressing Aspergillusaculeatusβ-glucosidase 1 for efficient biomass conversion. Biotechnol. Bioeng. 2012, 109, 92–99.
- Treebupachatsakul, T.; Shioya, K.; Nakazawa, H.; Kawaguchi, T.; Shioya, K.; Shida, Y.; Marikawa, Y.; Ogasawara, W.; Okada, H. Utilization of recombinant Trichodermareesei expressing Aspergillusaculeatus β-Glucosidase 1 (JN11) for a more economical production of ethanol from lignocellulosic biomass. J. Biosci. Bioeng. 2015, 120, 657–665.
- Dashtban, M.; Qin, W. Over expression of an exotic thermotolerant β-glucosidase in Trichodermareesei and its significant increase in cellulolytic activity and saccharification of barley straw. Microb. Cell Fact. 2012, 11, 63.
- Nigam, P.S. Microbial Enzymes with Special Characteristics for Biotechnological Applications. Biomolecules2013, 3, 597–611.
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic properties, functional attributes and industrial applications of b-glucosidases. 3 Biotech. 2016, 6.
- Ahmed, A.; Nasim, F.U.; Batool, K.; Bibi, A. Microbial β-Glucosidase: Sources, Production and Applications. J. Appl. Environ. Microbiol. 2017, 5, 31–46.
- Chotinsky, D. The use of enzymes to improve utilization of nutrient in poultry diets. Bulg. J. Agric. Sci. 2015, 21, 429–435.
- de Carvalho, J.L.M.; de Castro, I.M.; da Silva, C.A.B. A study of retention of sugars in the process of clarification of pineapple juice (Ananas comosus, L. Merril) by micro-and ultra-filtration. J. Food Eng. 2008, 87, 447–454.
- Ramadan, M.F. Enzymes in Fruit Juice Processing. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Kuddus, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-12-813280-7.
- Serra Colomer, M.; Funch, B.; Forster, J. The raise of Brettanomyces yeast species for beer production. Curr. Opin. Biotechnol. 2019, 56, 30–35.
- Annette, S.; Mette, L.; Peter, S.L.; Birgitte, K.A. Fungal Beta-Glucosidases: A Bottleneck in Industrial Use of Lignocellulosic Materials. Biomolecules 2013, 3, 612–631.
- Abdellaa, A.; Mazeeda, T.E.-S.; -Baz, A.F.E.; Yang, S.-T. Production of β-glucosidase from wheat bran and glycerol by Aspergillus niger in stirred tank and rotating fibrous bed bioreactors. Process. Biochem. 2016, 51, 1331–1337.
- Zhenming, C.; Zhe, C.; Guanglei, L.; Fang, W.; Liang, J.; Tong, Z. Saccharomycopsis fibuligera and its applications in biotechnology. Biotechnol. Adv. 2009, 27, 423–443.
- Goswami, S.; Gupta, N.; Datta, S. Using the β-glucosidase catalyzed reaction product glucose to improve the ionic liquid tolerance of β-glucosidases. Biotechnol. Biofuels 2016, 9, 72.
- Jalak, J.; Väljamäe, P. Mechanism of initial rapid rate retardation in cellobiohydrolase catalyzed cellulose hydrolysis. Biotechnol. Bioeng. 2010, 106, 871–883.
- Igarasi, K.; Uchihashi, T.; Koivula, A.; Wada, M.; Kimura, S.; Okamoto, T.; Penttila, M.; Ando, T.; Samejima, M. Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface. Science 2011, 333, 1279–1282.
- Teugjas, H.; Väljamäe, P. Selecting β-glucosidases to support cellulases in cellulose saccharification. Biotechnol. Biofuels 2013, 6, 105.
- Cao, L.; Wang, Z.; Ren, G.; Kong, W.; Li, L.; Xie, W.; Liu, Y. Engineering a novel glucose-tolerant β-glucosidase as supplementation to enhance the hydrolysis of sugarcane bagasse at high glucose concentration. Biotechnol. Biofuels 2015, 8, 202.
- Sharma, S.; Vaid, S.; Bhat, B.; Singh, S.; Bajaj, B.K. Thermostable Enzymes for Industrial Biotechnology. In Advances in Enzyme Technology; Singh, R., Singhania, R.R., Pandey, A., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 469–495.
- Pei, X.Q.; Yi, Z.L.; Tang, C.G.; Wu, Z.L. Three amino acid changes contribute markedly to the thermostability of β-glucosidase BglC from Thermobifida fusca. Bioresour. Technol. 2011, 102, 3337–3342.
- Banerjee, G.; Scott-Craig, J.S.; Walton, J.D. Improving enzymes for biomass conversion: A basic research perspective. Bioenergy Res. 2010, 3, 82–92.
- Banerjee, S.; Mudliar, S.; Sen, R.; Giri, B.; Satpute, D.; Chakrabarti, T.; Pandey, R.A. Commercializing lignocellulosic bioethanol: Technology bottlenecks and possible remedies. Biofuels Bioprod. Biorefin. 2010, 4, 77–93.
- Chen, H.L.; Chen, Y.C.; Lu, M.Y.; Chang, J.J.; Wang, H.T.; Ke, H.M.; Wang, T.Y.; Ruan, S.K.; Wang, T.Y.; Hung, K.Y.; et al. A highly efficient β-glucosidase from the buffalo rumen fungus Neocallimastix patriciarum W5. Biotechnol. Biofuels 2012, 5, 24.
- Lee, W.H.; Nan, H.; Kim, H.J.; Jin, Y.S. Simultaneous saccharification and fermentation by engineered Saccharomyces cerevisiae without supplementing extracellular β-glucosidase. J. Biotechnol. 2013, 167, 316–322.
- da Gama Ferreira, R.; Azzoni, A.R.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 81.
- Zahoor, S.; Javed, M.M.; Aftab, S.; Latif, F.; ul-Haq, I. Metabolic engineering and thermodynamic characterization of an extracellular β-glucosidase produced by Aspergillus niger. Afr. J. Biotechnol. 2011, 10, 8107–8116.
- Liu, Y.; Li, R.; Wang, J.; Zhang, X.; Jia, R.; Gao, Y.; Peng, H. Increased enzymatic hydrolysis of sugarcane bagasse by a novel glucose- and xylose-stimulated β-glucosidase from Anoxybacillus flavithermus subsp. yunnanensis E13. BMC Biochem. 2017, 18, 4.
- Yan, F.Y.; Xia, W.; Zhang, X.X.; Chen, S.; Nie, X.Z.; Qian, L. Characterization of β-glucosidase from Aspergillus terreus and its application in the hydrolysis of soybean isoflavones. J. Zhejiang Univ. Sci. B 2016, 17, 455–464.
- Liu, D.; Zhang, R.; Yang, X.; Zhang, Z.; Song, S.; Miao, Y.; Shen, Q. Characterization of a thermostable β-glucosidase from Aspergillus fumigatus Z5, and its functional expression in Pichia pastoris X33. Microb. Cell Factories 2012, 11, 25.
- Zhang, Z.; Wang, M.; Gao, R.; Yu, X.; Chen, G. Synergistic effect of thermostable β-glucosidase TN0602 and cellulase on cellulose hydrolysis. 3 Biotech 2017, 7, 54.
- Fusco, F.A.; Fiorentino, G.; Pedone, E.; Contursi, P.; Bartolucci, S.; Limauro, D. Biochemical characterization of a novel thermostable β-glucosidase from Dictyoglomus turgidum. Int. J. Biol. Macromol. 2018, 113, 783–791.
- Rapp, P. 1, 3-b-glucanase, 1, 6-b-glucanase and b-glucosidase activities of Sclerotium glucanicum, synthesis and properties. J. Gen. Microbiol. 1989, 135, 2847–2858.
- Zeng, Y.C.; Zhang, S.Z. Purification and properties of a b-glucosidase from Aspergillus phoenicis. Wei Sheng Wu Xue Bao 1989, 29, 195–199.
- Yeoman, C.J.; Han, Y.; Dodd, D.; Schroeder, C.M.; Mackie, R.I.; Cann, I.K.O. Thermostable Enzymes as Biocatalysts in the Biofuel Industry. Adv. Appl. Microbiol. 2010, 70, 1–55.
- Paramjeet, S.; Manasa, P.; Korrapati, N. Biofuels: Production of fungal-mediated ligninolytic enzymes and the modes of bioprocesses utilizing agro-based residues. Biocatal. Agric. Biotechnol. 2018, 14, 57–71.
- Arnthong, J.; Chuaseeharonnachai, C.; Boonyuen, N.; Tachaapaikun, C.; Chimchana, D.; Eurwilaichitr, L.; Champreda, V.; Chantasingh, D. Cooperative Decomposition of Rice Straw by Co-cultivation of Cellulolytic Fungi. J. Sci. 2018, 45, 645–652.
- Benoit-Gelber, I.; Gruntjes, T.; Vinck, A.; van Veluw, J.G.; Wösten, H.A.B.; Boeren, S.; Vervoort, J.J.M.; de Vries, R.P. Mixed colonies of Aspergillus niger and Aspergillus oryzae cooperatively degrading wheat bran. Fungal Genet. Biol. 2017, 102, 31–37.
- Chen, Z.; Lu, D.; Hao, F. Mixed culture of recombinant Trichodermareesei and Aspergillusniger for cellulase production to increase the cellulose degrading capability. Biomass Bioenergy 2018, 112, 93–98.
- Hu, H.L.; van den Brink, J.; Gruben, B.S.; Wosten, H.A.B.; Gu, J.-D.W.; de Vries, R.P. Improved enzyme production by co-cultivation of Aspergillusniger and Aspergillusoryzae and with other fungi. Int. J. Biodeterior. Biodegrad. 2011, 65, 248–252.
- Julieta, M.; Leandro, P.; Laura, L. Characterization of β-Glucosidase Produced by the White Rot Fungus Flammulina velutipes. J. Microbiol. Biotechnol. 2014, 25, 57–65.
- Oh, J.M.; Lee, J.P.; Baek, S.C.; Kim, S.G.; Jo, Y.D.; Kim, J.; Kim, H. Characterization of two extracellular β-glucosidases produced from the cellulolytic fungus Aspergillus sp. YDJ216 and their potential applications for the hydrolysis of flavone glycosides. Int. J. Biol. Macromol. 2018, 111, 595–603.
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high β-glucosidase activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155.
- Fan, G.; Xu, Y.; Zhang, X.; Lei, S.; Yang, S.; Pan, S. Characteristics of immobilised b-glucosidase and its effect on bound volatile compounds in orange juice. Int. J. Food Sci. Technol. 2011, 46, 2312–2320.
- Su, E.; Xia, T.; Gao, L.; Dai, Q.; Zhang, Z. Immobilization of bglucosidase and its aroma-increasing effect on tea beverage. Food Bioprod. Process. 2010, 88, 83–89.
- González-Pombo, P.; Fariña, L.; Carrau, F.; Batista-Viera, F.; Brena, B.M. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process Biochem. 2011, 46, 385–389.
- Hati, S.; Vij, S.; Singh, B.P.; Mandal, S. b-Glucosidase activity and bioconversion of isoflavones during fermentation of soymilk. J. Sci. Food Agric. 2015, 95, 216–220.
- Marazza, J.A.; Garro, M.S.; Savoy de Giori, G. Aglycone production by Lactobacillus rhamnosus CRL981 during soymilk fermentation. Food Microbiol. 2009, 26, 333–339.
- Wojtusik, M.; Yepes, C.M.; Villar, J.C.; Cordes, A.; Arroyo, M.; Garcia-Ochoa, F.; Ladero, M. Kinetic modeling of cellobiose by a β-glucosidase from Aspergillus fumigatus. Chem. Eng. Res. Des. 2018, 136, 502–512.
- Terry, D.B. Iminosugar Inhibitors of Substrate Reduction Therapy for Lysosomal Glycosphingolipidoses. In Iminosugars: From Synthesis to Therapeutic Application; Compain, P., Martin, O.R., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 249–268.
- Schröder, C.; Elleuche, S.; Blank, S.; Antranikian, G. Characterization of a heat-active archaeal β-glucosidase from a hydrothermal spring metagenome. Enzym. Microb. Technol. 2014, 57, 48–54.
- Elliston, A.; Collins, S.R.A.; Wilson, D.R.; Roberts, I.N.; Waldron, K.W. High concentrations of cellulosic ethanol achieved by fed batch semi simultaneous saccharification and fermentation of waste-paper. Bioresour. Technol. 2013, 134, 117–126.
- Lee, C.K.; Ibrahim, D.; Omar, I.C. Enzymatic deinking of various types of waste paper: Efficiency and characteristics. Proces Biochem. 2013, 48, 299–305.
- Elliston, A.; Collins, S.R.A.; Faulds, C.B.; Roberts, I.N.; Waldron, K.W. Biorefining of Waste Paper Biomass: Increasing the Concentration of Glucose by Optimising Enzymatic Hydrolysis. Appl. Biochem. Biotechnol.2014, 172, 3621–3634.
- Javed, M.; Buthe, A.; Rashid, M.; Wang, P. Cost-efficient entrapment of b-glucosidase in nanoscale latex and silicone polymeric thin films for use as stable biocatalysts. Food Chem. 2016, 190, 1078–1085.
