Animals obtain nutrition and energy by eating and to achieve a balance between growth and body health. When the nutrient intake is abnormal, the growth status changes, presumably resulting in changes in the intrinsic immune system. The zebrafish (Danio rerio), a well-known fish model organism, can serve as a suitable model to explore the changes happened to the innate immune system. In this study, the zebrafish underwent 3 weeks of fasting and refeeding for 3 to 7day periods. During this period, zebrafish displayed a specific growth phenomenon so called compensatory growth (CG), accompanied by increased susceptibility to pathogens after starvation. The kidneys suffering starvation displayed an increase of the amount of melano-macrophage centers and appeared oxidative stress, and the antioxidant enzymes activity like CAT, GSH-Px and SOD increased after fasting. In addition, the activity of ALP and lysozyme as well as il-1β mRNA expression enhanced after starvation. Taken together, oxidative stress caused by starvation and the danger-associated molecular patterns produced by injured renal tubules may have contributed to inflammation. This study showed that the function of the innate immune system in zebrafish could be influenced by nutrition status. Further study is needed to explore how starvation increases susceptibility and how the metabolic state influences different kinds of immune cells.
- innate immune system
- kidney
- oxidative stress
- compensatory growth
- zebrafish
1. Introduction
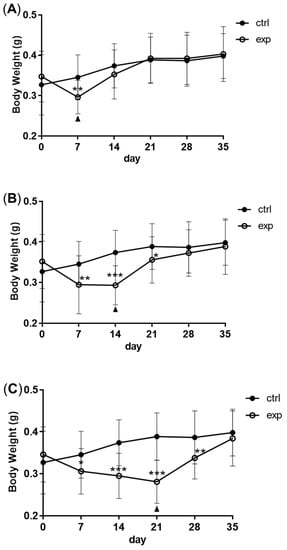
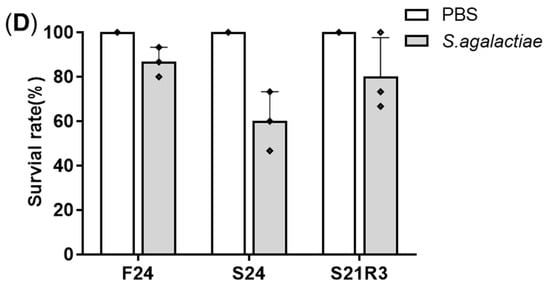
2.Changes in Body Weight and Antibacterial Properties of Zebrafish during Fasting and Refeeding
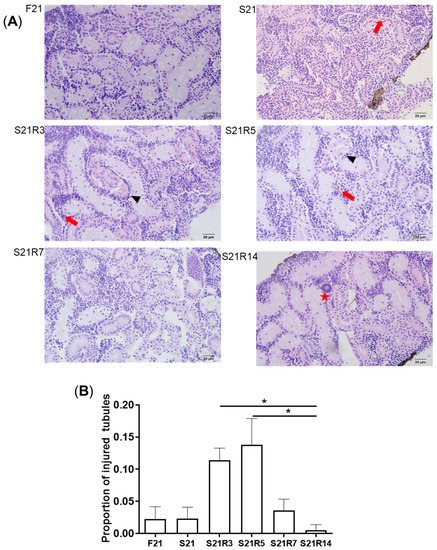
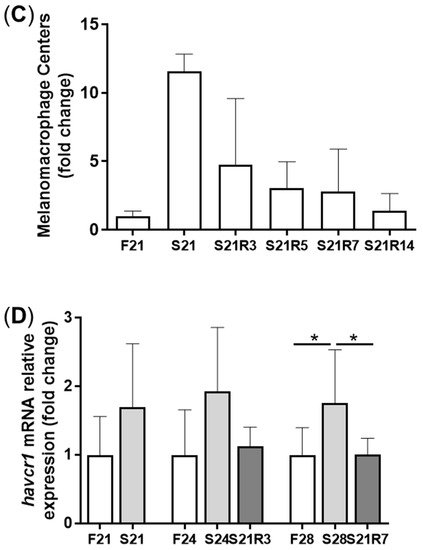
3. Morphologic Alterations of the Kidneys of Zebrafish during Fasting and Refeeding
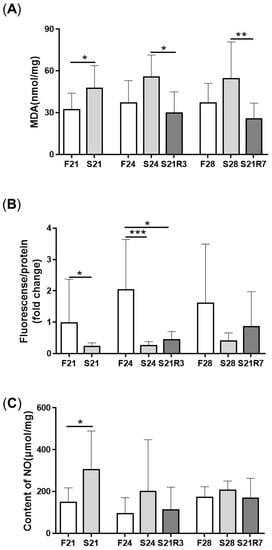
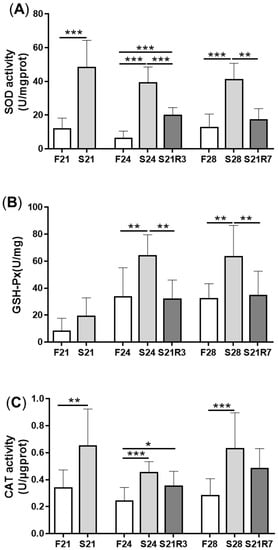
4. Oxidative Stress Was Triggered during Fasting and Refeeding
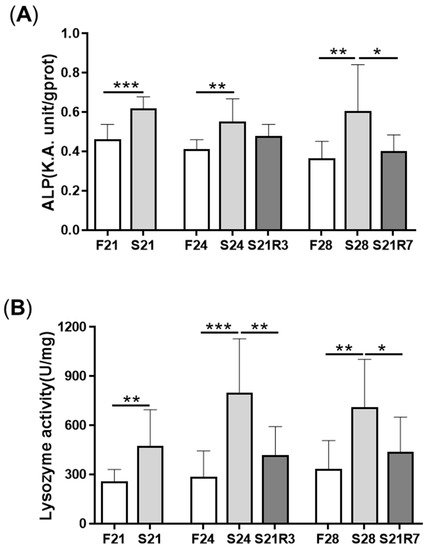
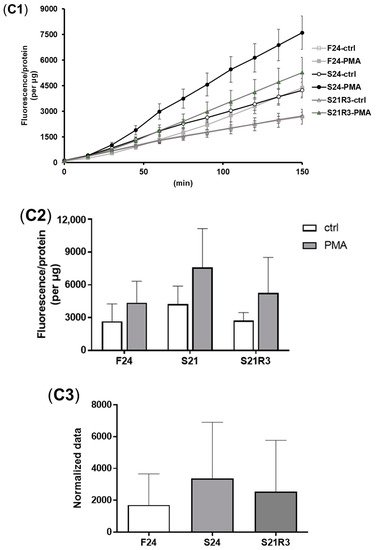
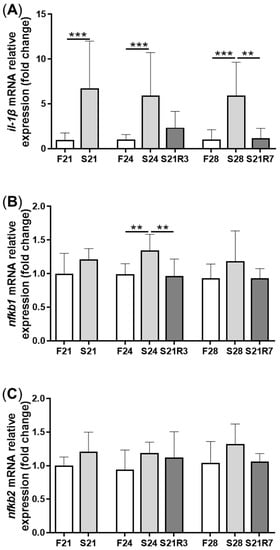
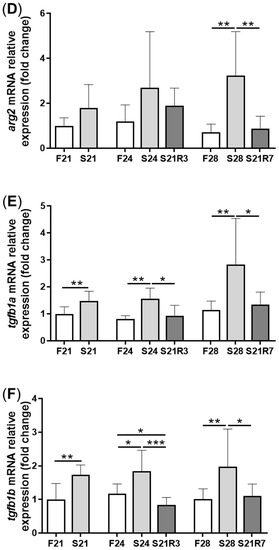
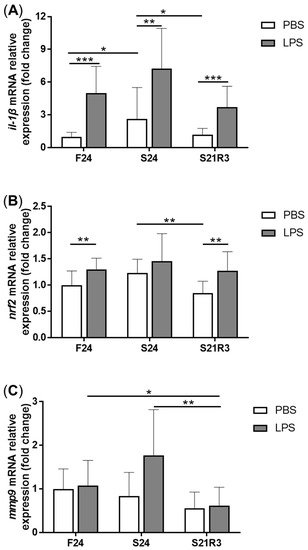
5. The Innate Immune System of the Zebrafish Kidney Was Affected by Fasting and Refeeding
References
- Alwarawrah, Y.; Kiernan, K.; Maciver, N.J. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018, 9, 1055.
- Wu, Z.; Isik, M.; Moroz, N.; Steinbaugh, M.J.; Zhang, P.; Blackwell, T.K. Dietary Restriction Extends Lifespan through Metabolic Regulation of Innate Immunity. Cell Metab. 2019, 29, 1192–1205.e8.
- Wilson, P.N.; Osbourn, D.F. Compensatory growth after undernutrition in mammals and birds. Biol. Rev. 1960, 35, 324–363.
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish. Fish. 2003, 4, 147–190.
- Bilton, H.T.; Robins, G.L. The Effects of Starvation and Subsequent Feeding on Survival and Growth of Fulton Channel Sockeye Salmon Fry (Oncorhynchus nerka). J. Fish. Res. Board Can. 1973, 30, 1–5.
- Won, E.T.; Borski, R.J. Endocrine regulation of compensatory growth in fish. Front. Endocrinol. 2013, 4, 74.
- Jobling, M. Are compensatory growth and catch-up growth two sides of the same coin? Aquac. Int. 2009, 18, 501–510.
- Whyte, S.K. The innate immune response of finfish—A review of current knowledge. Fish. Shellfish. Immunol. 2007, 23, 1127–1151.
- Novoa, B.; Figueras, A. Zebrafish: Model for the Study of Inflammation and the Innate Immune Response to Infectious Diseases. Curr. Top. Innate Immun. 2012, 946, 253–275.
- Mathis, D.; Shoelson, S.E. Foreword Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011, 11, 81–83.
- Walrand, S.; Moreau, K.; Caldefie, F.; Tridon, A.; Chassagne, J.; Portefaix, G.; Cynober, L.; Beaufrère, B.; Vasson, M.-P.; Boirie, Y. Specific and nonspecific immune responses to fasting and refeeding differ in healthy young adult and elderly persons. Am. J. Clin. Nutr. 2001, 74, 670–678.
- Oarada, M.; Kamei, K.; Gonoi, T.; Tsuzuki, T.; Toyotome, T.; Hirasaka, K.; Nikawa, T.; Sato, A.; Kurita, N. Beneficial effects of a low-protein diet on host resistance to Paracoccidioides brasiliensis in mice. Nutrition 2009, 25, 954–963.
- Morris, H.J.; Carrillo, O.V.; Llauradó, G.; Alonso, M.E.; Bermúdez, R.C.; Lebeque, Y.; Fontaine, R.; Soria, N.E.; Venet, G. Effect of starvation and refeeding on biochemical and immunological status of Balb/c mice: An experimental model of malnutrition. Immunopharmacol. Immunotoxicol. 2010, 33, 438–446.
- Petersen, P.S.; Lei, X.; Seldin, M.M.; Rodriguez, S.; Byerly, M.S.; Wolfe, A.; Whitlock, S.; Wong, G.W. Dynamic and extensive metabolic state-dependent regulation of cytokine expression and circulating levels. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R1458–R1470.
- Li, T.; Qi, M.; Gatesoupe, F.-J.; Tian, D.; Jin, W.; Li, J.; Lin, Q.; Wu, S.; Li, H. Adaptation to Fasting in Crucian Carp (Carassius auratus): Gut Microbiota and Its Correlative Relationship with Immune Function. Microb. Ecol. 2018, 78, 6–19.
- Martin, S.A.M.; Douglas, A.; Houlihan, D.F.; Secombes, C.J. Starvation alters the liver transcriptome of the innate immune response in Atlantic salmon (Salmo salar). BMC Genom. 2010, 11, 418.
- Najafi, A.; Salati, A.P.; Yavari, V.; Asadi, F. Effects of short term fasting and refeeding on some hematological and immune parameters in Mesopotamichthys sharpeyi (Gunther, 1874) fingerlings. Iran J. Sci. Technol. A 2015, 39, 383–389.
- Gimbo, R.Y.; Favero, G.C.; Montoya, L.N.F.; Urbinati, E.C. Energy deficit does not affect immune responses of experimentally infected pacu (Piaractus mesopotamicus). Fish. Shellfish. Immunol. 2015, 43, 295–300.
- Caruso, G.; Denaro, M.G.; Caruso, R.; Mancari, F.; Genovese, L.; Maricchiolo, G. Response to short term starvation of growth, haematological, biochemical and non-specific immune parameters in European sea bass (Dicentrarchus labrax) and blackspot sea bream (Pagellus bogaraveo). Mar. Environ. Res. 2011, 72, 46–52.
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. Anais Acad. Brasileira Ciências 2018, 90, 3403–3414.
- Gill, R.; Tsung, A.; Billiar, T.R. Linking oxidative stress to inflammation: Toll-like receptors. Free. Radic. Biol. Med. 2010, 48, 1121–1132.
- Li, L.; Chen, Y.; Gibson, S.B. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell. Signal. 2013, 25, 50–65.
- Wu, C.-A.; Chao, Y.; Shiah, S.-G.; Lin, W.-W. Nutrient deprivation induces the Warburg effect through ROS/AMPK-dependent activation of pyruvate dehydrogenase kinase. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1833, 1147–1156.
- Renshaw, S.A.; Trede, N.S. A model 450 million years in the making: Zebrafish and vertebrate immunity. Dis. Model. Mech. 2012, 5, 38–47.
- Vaidya, V.S.; Ozer, J.S.; Dieterle, F.; Collings, F.B.; Ramirez, V.; Troth, S.; Muniappa, N.; Thudium, D.; Gerhold, D.; Holder, D.J.; et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 2010, 28, 478–485.
- Yin, W.; Naini, S.M.; Chen, G.; Hentschel, D.M.; Humphreys, B.D.; Bonventre, J.V. Mammalian Target of Rapamycin Mediates Kidney Injury Molecule 1-Dependent Tubule Injury in a Surrogate Model. J. Am. Soc. Nephrol. 2015, 27, 1943–1957.
- Craig, P.M.; Moon, T.W. Fasted zebrafish mimic genetic and physiological responses in mammals: A model for obesity and diabetes? Zebrafish 2011, 8, 109–117.
- Jia, J.; Zhang, Y.; Yuan, X.; Qin, J.; Yang, G.; Yu, X.; Wang, B.; Sun, C.; Li, W. Reactive oxygen species participate in liver function recovery during compensatory growth in zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2018, 499, 285–290.
- Luo, Z.-W.; Jiang, Y.-H.; Lin, L.-B.; Deng, X.-Y.; Zhang, Q. Genome-wide differential expression analysis explores antibacterial molecular mechanisms of zebrafish intestine upon pathogenic Streptococcus agalactiae challenge. Aquac. Rep. 2021, 19, 100639.

