Synthesis of BA
BA are predominantly produced in the liver, via two biosynthetic pathways: The classical (or neutral) pathway and the alternative (or acidic) pathway [7] (Figure 1). The synthesis of BA from cholesterol involves at least 16 enzymes [8]. The classical pathway is initiated by cytochrome P450 7A1 (CYP7A1), also known as cholesterol 7α hydroxylase. Cholesterol is converted to 7α-hydroxycholesterol by CYP7A1, which is the rate-limiting enzyme in this pathway. 7α-hydroxycholesterol is then converted to 7α-Hydroxy-4-cholesten-3-one. Cytochrome P450 8B1 (CYP8B1), also known as sterol 12α-hydroxylase, is responsible for the production of CA from 7α-hydroxy-4-cholesten-3-one, but 7α-hydroxy-4-cholesten-3-one is also converted to CDCA by cytochrome P450 27A1 (CYP27A1), also known as sterol 27-hydroxylase. The alternative pathway begins with the conversion of cholesterol to (25R)-26-hydroxycholesterol [9] by CYP27A1. Cytochrome P450 7B1 (CYP7B1), also known as oxysterol 7-α-hydroxylase, leads (25R)-26-hydroxycholesterol to CDCA. CDCA is converted to α-muricholic acid (MCA), and β-MCA. These BA are then conjugated with glycine or taurine. BA synthesized in the liver are referred to as “primary BA” [10,11,12]. Primary BA in rodents are also conjugated with taurine in the liver [13]. Bile salt export pump (BSEP) and multidrug resistance-associated protein 2 (MRP2) are the transporters that mediate the secretion of BA from hepatocytes into hepatic bile canaliculi (Figure 2). BSEP is the main transporter of BA, whereas MRP2 principally transports BA glucuronides and sulfates [1,14].
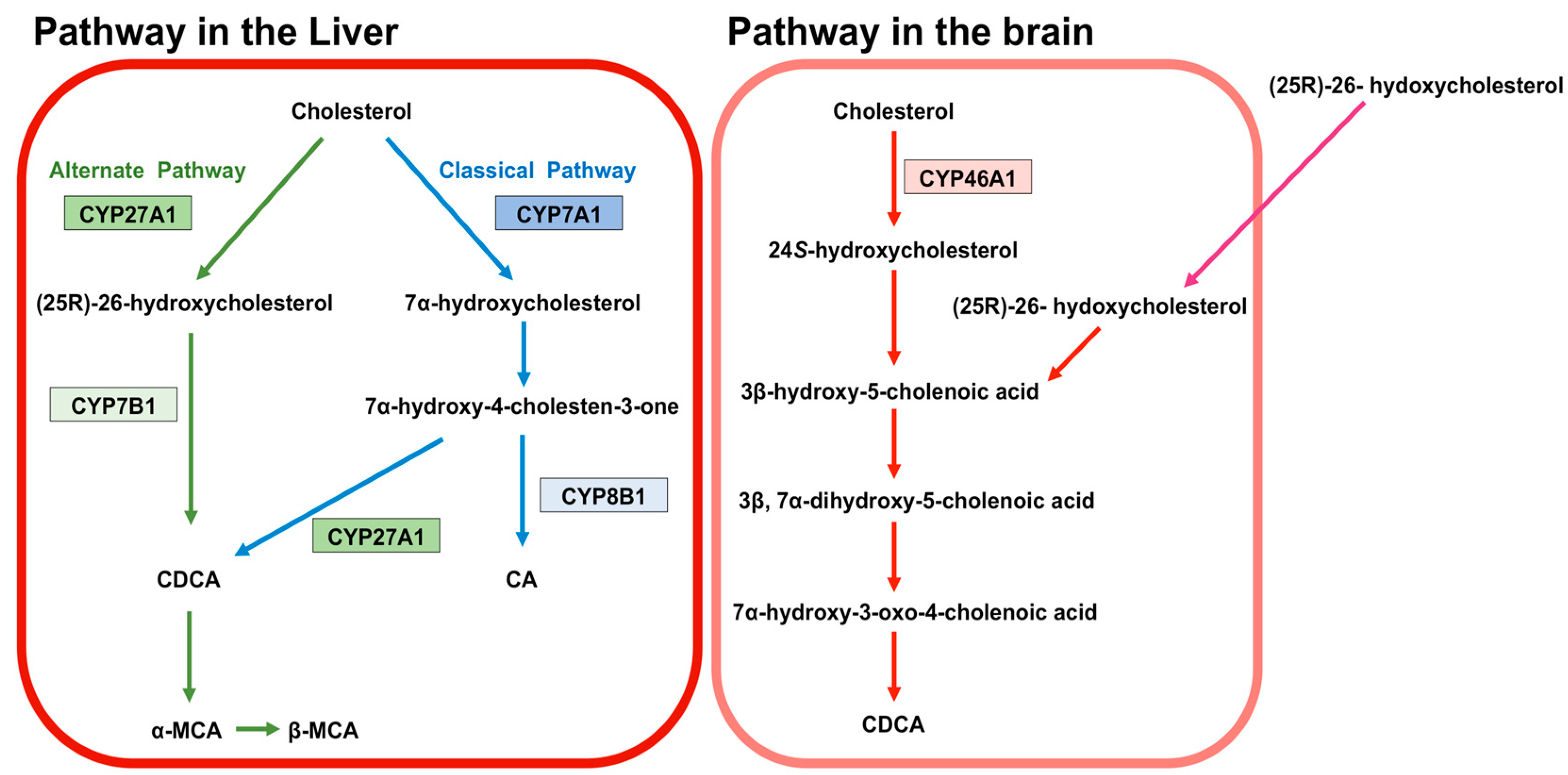
Figure 1. Synthesis of bile acids (BA). The classical pathway is initiated by cytochrome P450 7A1 (CYP7A1). CYP7A1 converts cholesterol to 7α-hydroxycholesterol. 7α-hydroxycholesterol is then converted to 7α-hydroxy-4-cholesten-3-one. Cytochrome P450 8B1 (CYP8B1) leads the production of CA from 7α-hydroxy-4-cholesten-3-one. 7α-hydroxy-4-cholesten-3-one is also converted to CDCA by cytochrome P450 27A1 (CYP27A1). The alternative pathway begins with converting cholesterol to (25R)-26-hydroxycholesterol by CYP27A1. Cytochrome P450 7B1 (CYP7B1) leads (25R)-26-hydroxycholesterol to CDCA. CDCA is converted to α-muricholic acid (MCA), and β-MCA. These BA are then conjugated with glycine or taurine. BA in rodents are also conjugated with taurine in the liver. BA synthesized in the liver are called primary BA. In the brain, 24S-hydroxycholesterol is converted from cholesterol by cytochrome P450 46A1 (CYP46A1). 24S-hydroxycholesterol is a precursor of 3β-hydroxy-5-cholenoic acid, which can be converted to CDCA through the intermediates (3β, 7α-dihydroxy-5-cholenoic acid and 7α-hydroxy-3-oxo-4-cholenoic acid). A large amount of (25R)-26-hydoxycholesterol incorporates to brain from circulation, and (25R)-26-hydoxycholesterol can also be converted to 3β-hydroxy-5-cholenoic acid.
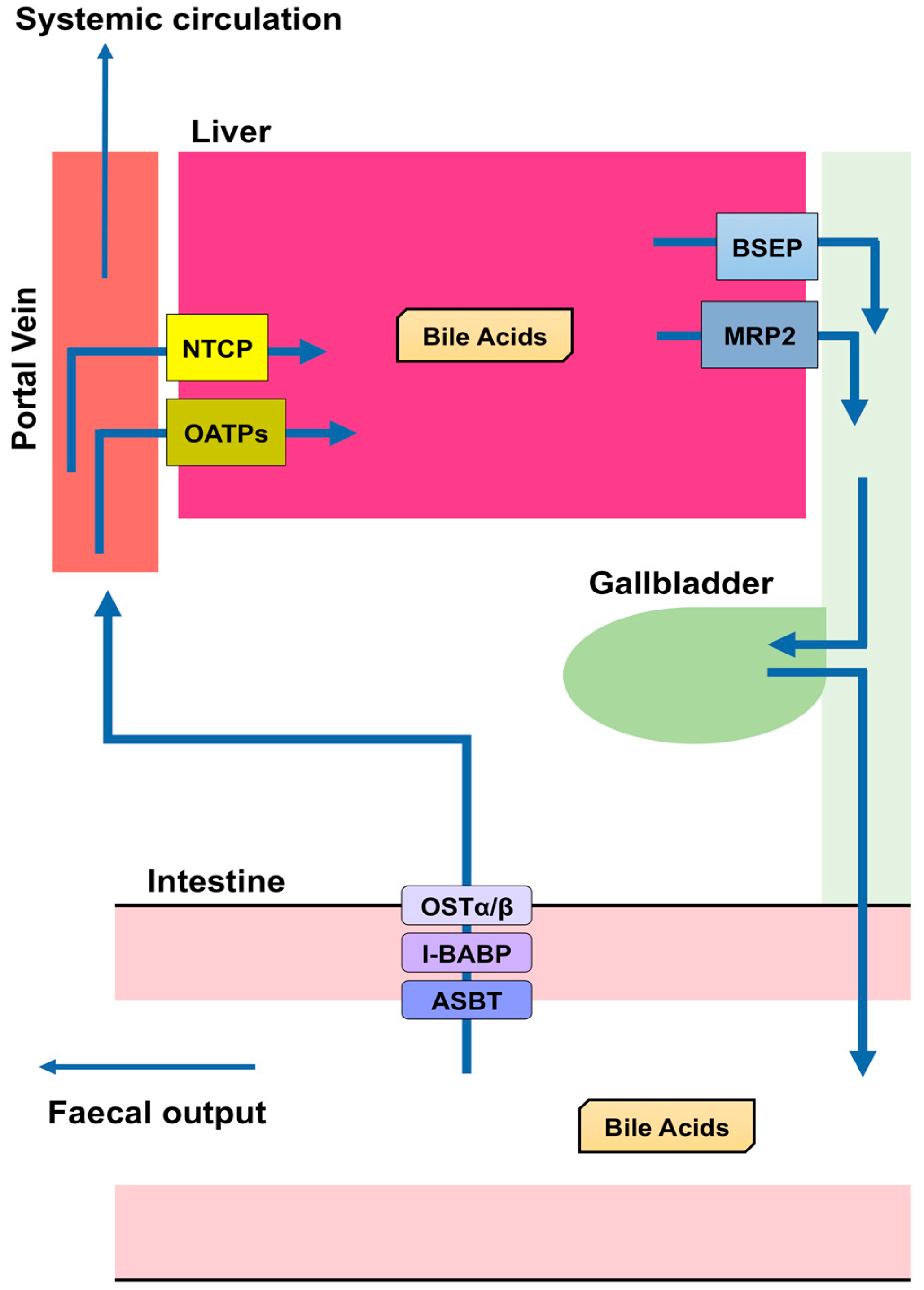
Figure 2. Enterohepatic circulation. Bile salt export pump (BSEP) and multidrug resistance-associated protein 2 (MRP2) are transporters mediating the secretion of bile acids (BA) from hepatocytes to the bile canaliculus in the liver. BA are stored in the gallbladder and secreted into the small intestine after a meal. Most BA (approximately 95%) are reabsorbed, whereas the remainder is excreted with feces. Apical sodium dependent bile acid transporter (ASBT) in the apical brush border of enterocytes takes BA into enterocytes. Ileal bile acid-binding protein (I-BABP) is related to the intracellular transport in enterocytes. The passage of BA through the basolateral membrane of enterocytes into the portal blood occurs through organic solute transporter (OST) α and β. BA released into the blood from the small intestine are transported into the liver by Na+-taurocholate co-transporting polypeptide (NTCP) or organic anion-transporting polypeptides (OATPs). These BA are then reconjugated and secreted with newly produced BA. This recycling system is termed enterohepatic circulation.
BA in the Brain
References
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678–693.
- Hofmann, A.F.; Hagey, L.R.; Krasowski, M.D. Bile salts of vertebrates: Structural variation and possible evolutionary significance. J. Lipid Res. 2010, 51, 226–246.
- Reschly, E.J.; Ai, N.; Ekins, S.; Welsh, W.J.; Hagey, L.R.; Hofmann, A.F.; Krasowski, M.D. Evolution of the bile salt nuclear receptor FXR in vertebrates. J. Lipid Res. 2008, 49, 1577–1587.
- Molinaro, A.; Wahlstrom, A.; Marschall, H.U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018, 29, 31–41.
- Mano, N.; Goto, T.; Uchida, M.; Nishimura, K.; Ando, M.; Kobayashi, N.; Goto, J. Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. J. Lipid Res. 2004, 45, 295–300.
- Zheng, X.; Chen, T.; Zhao, A.; Wang, X.; Xie, G.; Huang, F.; Liu, J.; Zhao, Q.; Wang, S.; Wang, C.; et al. The Brain Metabolome of Male Rats across the Lifespan. Sci Rep. 2016, 6, 24125.
- Chiang, J.Y. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966.
- Russell, D.W. Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res. 2009, 50, S120–125.
- Fakheri, R.J.; Javitt, N.B. 27-Hydroxycholesterol, does it exist? On the nomenclature and stereochemistry of 26-hydroxylated sterols. Steroids 2012, 77, 575–577.
- De Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab.2013, 17, 657–669.
- Bathena, S.P.; Mukherjee, S.; Olivera, M.; Alnouti, Y. The profile of bile acids and their sulfate metabolites in human urine and serum. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 942–943, 53–62.
- Nakashima, T.; Sano, A.; Seto, Y.; Nakajima, T.; Nakagawa, Y.; Okuno, T.; Takino, T.; Hasegawa, T. Unusual trihydroxy bile acids in the urine of healthy humans. Clin. Chim. Acta 1986, 160, 47–53.
- Li, T.; Apte, U. Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. Adv. Pharmacol. 2015, 74, 263–302.
- Trauner, M.; Boyer, J.L. Bile salt transporters: Molecular characterization, function, and regulation. Physiol. Rev. 2003, 83, 633–671.
- Chikai, T.; Nakao, H.; Uchida, K. Deconjugation of bile acids by human intestinal bacteria implanted in germ-free rats. Lipids 1987, 22, 669–671.
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651.
- Lepercq, P.; Gerard, P.; Beguet, F.; Raibaud, P.; Grill, J.P.; Relano, P.; Cayuela, C.; Juste, C. Epimerization of chenodeoxycholic acid to ursodeoxycholic acid by Clostridium baratii isolated from human feces. FEMS Microbiol. Lett. 2004, 235.
- Martin, F.P.; Dumas, M.E.; Wang, Y.; Legido-Quigley, C.; Yap, I.K.; Tang, H.; Zirah, S.; Murphy, G.M.; Cloarec, O.; Lindon, J.C.; et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol. Syst. Biol. 2007, 3, 112.
- Fu, Z.D.; Csanaky, I.L.; Klaassen, C.D. Gender-divergent profile of bile acid homeostasis during aging of mice. PLoS ONE 2012, 7, e32551.
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212.
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099.
- Oelkers, P.; Kirby, L.C.; Heubi, J.E.; Dawson, P.A. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J. Clin. Invest. 1997, 99, 1880–1887.
- Meier, Y.; Eloranta, J.J.; Darimont, J.; Ismair, M.G.; Hiller, C.; Fried, M.; Kullak-Ublick, G.A.; Vavricka, S.R. Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab. Dispos. 2007, 35, 590–594.
- Shneider, B.L.; Dawson, P.A.; Christie, D.M.; Hardikar, W.; Wong, M.H.; Suchy, F.J. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J. Clin. Invest. 1995, 95, 745–754.
- Christie, D.M.; Dawson, P.A.; Thevananther, S.; Shneider, B.L. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am. J. Physiol. 1996, 271, G377–G385.
- Kiriyama, Y.; Nochi, H. Role and Cytotoxicity of Amylin and Protection of Pancreatic Islet β-Cells from Amylin Cytotoxicity. Cells 2018, 7, 95.
- Wali, J.A.; Masters, S.L.; Thomas, H.E. Linking metabolic abnormalities to apoptotic pathways in Beta cells in type 2 diabetes. Cells 2013, 2, 266–283.
- Tuomi, T.; Santoro, N.; Caprio, S.; Cai, M.; Weng, J.; Groop, L. The many faces of diabetes: A disease with increasing heterogeneity. Lancet 2014, 383, 1084–1094.
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083.
- Hansen, M.; Sonne, D.P.; Mikkelsen, K.H.; Gluud, L.L.; Vilsboll, T.; Knop, F.K. Bile acid sequestrants for glycemic control in patients with type 2 diabetes: A systematic review with meta-analysis of randomized controlled trials. J. Diabetes Complicat. 2017, 31, 918–927.
- Wu, Y.; Aquino, C.J.; Cowan, D.J.; Anderson, D.L.; Ambroso, J.L.; Bishop, M.J.; Boros, E.E.; Chen, L.; Cunningham, A.; Dobbins, R.L.; et al. Discovery of a highly potent, nonabsorbable apical sodium-dependent bile acid transporter inhibitor (GSK2330672) for treatment of type 2 diabetes. J. Med. Chem 2013, 56, 5094–5114.
- Lin, M.C.; Kramer, W.; Wilson, F.A. Identification of cytosolic and microsomal bile acid-binding proteins in rat ileal enterocytes. J. Biol. Chem. 1990, 265, 14986–14995.
- Christian, W.V.; Li, N.; Hinkle, P.M.; Ballatori, N. β-Subunit of the Ostalpha-Ostbeta organic solute transporter is required not only for heterodimerization and trafficking but also for function. J. Biol. Chem. 2012, 287, 21233–21243.
- Ballatori, N.; Christian, W.V.; Lee, J.Y.; Dawson, P.A.; Soroka, C.J.; Boyer, J.L.; Madejczyk, M.S.; Li, N. OSTalpha-OSTbeta: A major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 2005, 42, 1270–1279.
- Dawson, P.A.; Hubbert, M.; Haywood, J.; Craddock, A.L.; Zerangue, N.; Christian, W.V.; Ballatori, N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J. Biol. Chem. 2005, 280, 6960–6968.
- Ananthanarayanan, M.; Ng, O.C.; Boyer, J.L.; Suchy, F.J. Characterization of cloned rat liver Na(+)-bile acid cotransporter using peptide and fusion protein antibodies. Am. J. Physiol 1994, 267, G637–G643.
- Stieger, B.; Hagenbuch, B.; Landmann, L.; Hochli, M.; Schroeder, A.; Meier, P.J. In situ localization of the hepatocytic Na+/Taurocholate cotransporting polypeptide in rat liver. Gastroenterology 1994, 107, 1781–1787.
- Hagenbuch, B.; Scharschmidt, B.F.; Meier, P.J. Effect of antisense oligonucleotides on the expression of hepatocellular bile acid and organic anion uptake systems in Xenopus laevis oocytes. Biochem J. 1996, 316, 901–904.
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123.
- Csanaky, I.L.; Lu, H.; Zhang, Y.; Ogura, K.; Choudhuri, S.; Klaassen, C.D. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: Studies in Oatp1b2-null mice. Hepatology 2011, 53, 272–281.
