The mitogen-activated protein kinase (MAPK) is one such complex interconnected signaling cascade with frequent involvement in oncogenesis, tumor progression, and drug resistance. The MAPK family consists of a large number of kinases altered in cancers and against which many targeted therapies were developed pathways in response to experimental MAPK inhibition. The mitogen-activated protein kinase (MAPK) pathway is an important bridge in the switch from extracellular signals to intracellular responses. Alterations of signaling cascades are found in various diseases, including cancer, as a result of genetic and epigenetic changes.
The framework of a signaling cascade such as MAPK is complex, with many interacting pathways and constant crosstalk, which are each subjected to fine-tuning and switch-like activations of regulatory factors (Figure 1). As an overview, the MAPK pathways converge in the amplification of key molecules that sustain cell proliferation, growth, and survival processes. The outline of the MAPK signaling cascade consists of the interaction of one or more growth factors (GFs) with their specific growth factor receptors (GFRs).
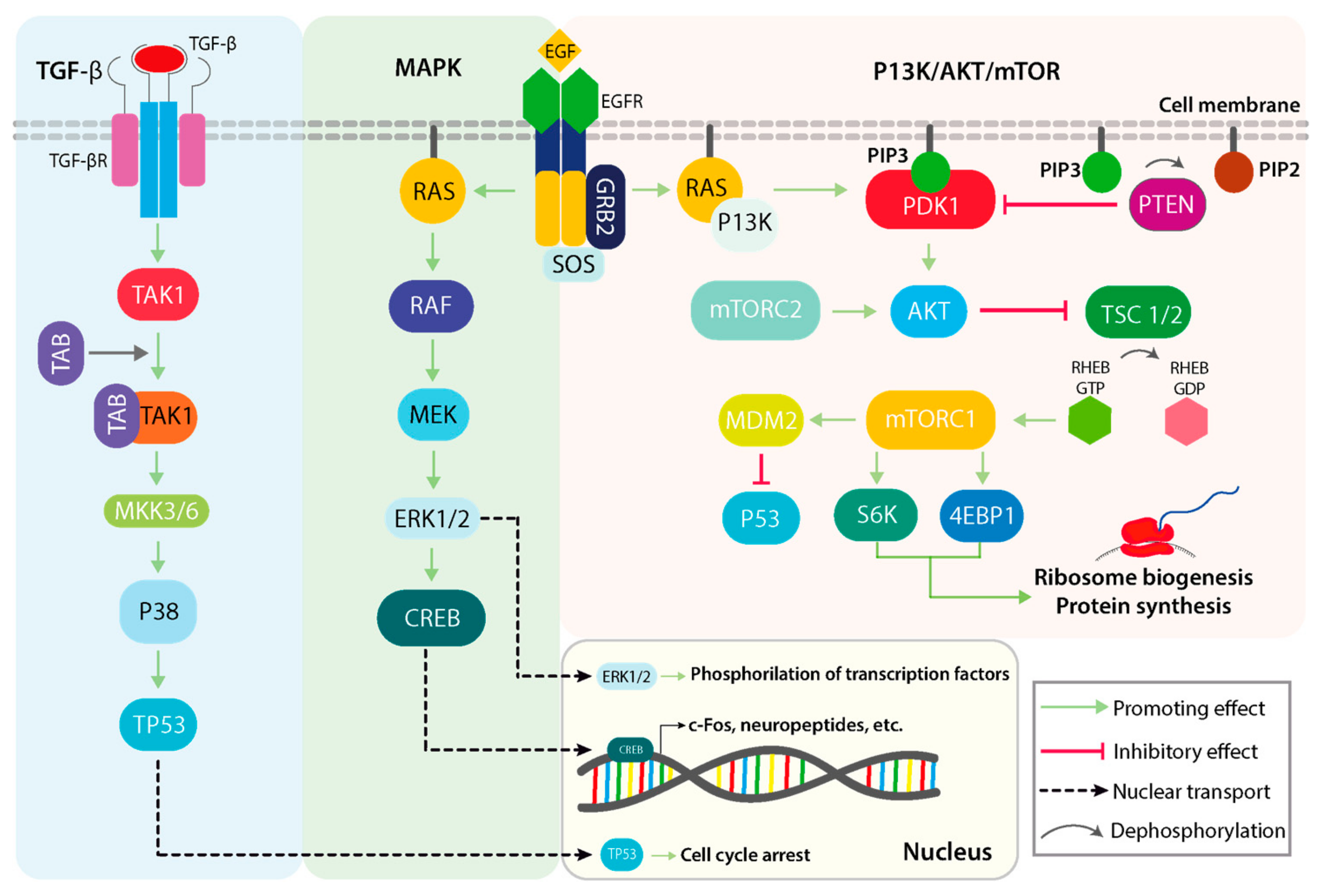
Figure 1. Parallel outline of several physiological roles of the TGFβ/p38, mitogen-activated protein kinase (MAPK), and P13k/AKT/mTOR signaling pathways. The p38 mitogen-activated kinase can be activated following upstream cytokine stimulation of the TGFβ pathway, which can subsequently activate TP53 in normal physiological conditions. TGFβ activation of p38 is not dependent on canonical SMAD signaling, but rather on the TAB/TAK1 complex and the MKK3/6 mitogen-activated protein kinase kinases. The canonical MAPK kinase pathway initiates with an extracellular stimulus in the form of growth factors (GFs) that bind and activate receptor tyrosine kinases (RTKs) on the cell membrane. Downstream activation of RAS, RAF and MEK in that order converge in the activation of the ERK1/2 transcription factor activator. The P13K/AKT/mTOR cascade can also be activated via RTKs and RAS, and its main implications are related to metabolic signaling and protein synthesis that sustain cell growth. TGFβ: transforming growth factor beta 1; p38: p38 kinase; P13k: phosphoinositide-3-kinase; AKT: v-akt murine thymoma viral oncogene homolog 1; mTOR: mechanistic target of rapamycin kinase; TAB: TGF-beta activated kinase 1 binding protein 2; TAK1: TGF-beta activated kinase 1; MKK3/6: mitogen-activated protein kinase kinase 3; RAS: small G-protein; RAF: Raf oncogene; MEK: MAP kinse-ERK kinase; RTKs: Receptor tyrosine kinases.
The first line of cytosolic intermediates that activate the phosphorylation cascade of the MAPK pathway are represented by the RAS superfamily of GTPases, which comprise over 150 small G-proteins, such as HRAS, KRAS, NRAS, and others. Subsequent to EGFR activation, the RAS GTPase is activated with the help of EGFR-associated of nucleotide exchange factor Son of Sevenless 1 (SOS1). SOS determines the rapid conversion of GTP to GDP, which is a limiting condition for the formation of RAS-GTP, the active form of RAS. RAF is the downstream effector of RAS and, thus, it is dependent on the interaction with an activated RAS. The RAF family includes several variants (e.g., ARAF, BRAF, CRAF), all of which consist of serine/threonine kinases responsible for the pathway progression by activating MEK (MAP kinse-ERK kinase) and ERK1/2 (Extracellular signal-regulated kinases). The activation cascade is in the following order: MAPKKK (Mitogen-activated protein kinase kinase kinases, represented by RAF and its variants), followed by MAPK kinase (MAPKK: MEK1/2/3/4/5/6/7), and finally the MAPK. There are three main classical MAPKs with different isoforms ERKs (with ERK1 and ERK2 isoforms), JNKs (c-Jun N-terminal kinases, with JNK1, JNK2, and JNK3 isoforms), and p38 MAPKs (with p38α, p38β, p38γ, and p38δ isoforms).
