Chronic obstructive pulmonary disease (COPD) is a major health problem with increasing incidence and mortality worldwide. There are remaining issues that are difficult to resolve with the current treatment algorithm. COPD patients face a number of unmet needs such as symptoms, exacerbations, physical inactivity, and loss of social activities. In this article, we categorize potential therapeutic targets from the viewpoint of pulmonary and systemic comorbid conditions,, and address recent data concerning the pathophysiological link with COPD and the impact of intervention for comorbid conditions, in order to obtain evidence that could enable us to provide personalized COPD management.
- COPD
- cardiovascular disease
- bronchiectasis
- frailty
- asthma
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a major health problem with increasing incidence and mortality worldwide [1,2]. The current mainstay of pharmacotherapy is bronchodilators, and recent advances in these agents have enabled further improvements in lung function and reductions in symptoms and exacerbations [3,4]. However, it has also become apparent that there are remaining issues that are difficult to resolve with the current treatment algorithm. COPD patients face a number of unmet needs such as symptoms, exacerbations, physical inactivity, and loss of social activities [5–10].
Most of the current treatment algorithms are based on the severity of symptoms and exacerbation history, and recommend the use of drugs other than inhalation medications when symptoms remain or exacerbations occur [3,11]. However, even a single exacerbation can accelerate the loss of lung function, inducing physical inactivity and increasing the risk of death [12,13]. A large-scale epidemiological study in Japan showed that dyspnea of the modified Medical Research Council (mMRC) grade 2 or more remains in almost one half of COPD patients managed by pulmonologists [14,15]. Given the burden of such unmet needs, it is very important to promote pre-emptive therapies that target treatable traits [5–9].
There are various risk factors and triggers for patients’ unmet needs, while the factors can be roughly divided into two categories (Figure 1). One is the usual characteristics of COPD patients, such as smoking and airflow obstruction. As a standard approach, it goes without saying that smoking cessation, bronchodilator, pulmonary rehabilitation, and vaccination are established treatments, and they should be included under general recommendations for all patients [3,11]. The other includes specific characteristics in patients with comorbid conditions, such as asthma, bronchiectasis, and cardiovascular disease [9]. These comorbidities, which are also associated with the diversity of COPD [5–9], can cause patients’ unmet needs to resist usual care. However, treatable conditions that are not recognized as therapeutic targets may be latent in patients with COPD. However, there is accumulating evidence indicating that some clinical features and specific biomarkers can be used to identify patients who should be given second controllers, such as inhaled corticosteroids (ICS) [16–18], selective β1-blockers [19–21], and macrolides [22–24]. We again realized that treatable traits should be assessed and treated as early as possible [9].
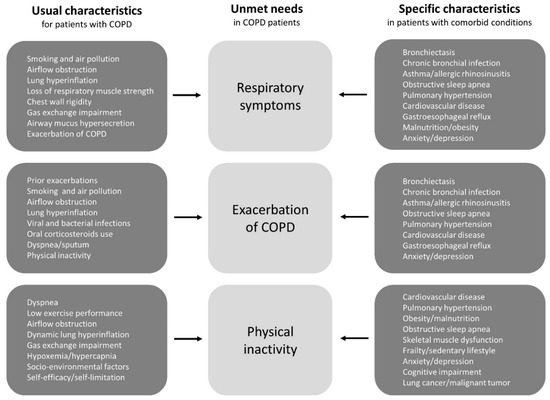
Figure 1. The risk factors and triggers for COPD patients’ unmet needs can be divided into two categories; one is the usual clinical characteristics of COPD patients and the other is the specific clinical characteristics of patients with comorbid conditions. Treatable conditions that are not recognized as therapeutic targets may be latent in patients with COPD. Abbreviations: COPD, chronic obstructive pulmonary disease.
2. Pulmonary Hypertension
Pulmonary hypertension (PH), which is one of the significant comorbidities in COPD, is associated with a worse prognosis [84]. Patients with a mean pulmonary artery pressure (mPAP) >20 mmHg and pulmonary capillary wedge pressure (PCWP) <15 mmHg with right heart catheterization are defined as PH [85]. The mechanisms of PH-COPD have been considered to be hypoxic pulmonary vasoconstriction, vascular injury, and reduction in the vascular beds, which is due to alveolar destruction by the inhalation of cigarette smoke and harmful substances [86]. Notably, PH-COPD is more prevalent and severe in COPD with pulmonary fibrosis compared to COPD without fibrosis [87,88].
Long-term oxygen therapy suppressed mPAP elevation, and may be effective for PH-COPD with respiratory failure [89,90]. Pulmonary vasodilators which are approved for pulmonary artery hypertension (PAH) may worsen the ventilation/perfusion mismatch and hypoxemia [91,92]. Although the efficacy of PAH-targeted therapy for PH-COPD is still controversial [91–94], a subgroup of PH-COPD who shows severe pulmonary hypertension despite mild airflow limitation may benefit from pulmonary vasodilators [95,96]. From the perspective of the heterogeneous pathogenesis and poor prognosis of PH patients with COPD, further and different targeted therapies are needed.
3. Cardiovascular Disease
COPD and cardiovascular disease (CVD) frequently coexist, and the relationship can be explained by shared risk factors, lung hyperinflation, loss of vascular capacity, oxidative stress, and systemic inflammation [97]. The prevalence of CVD in patients with COPD ranges from 20% to 70% [98], with 2.5 times greater odds of CVD in COPD patients compared with the non-COPD cohort [99]. The prevalence of COPD in patients with chronic heart failure (CHF) ranges from 9% to 52% [100], and the presence of COPD and rapid FEV1 decline are risk factors for CHF [101,102]. Arrhythmia is common in COPD patients, and reduced FEV1 is associated with incident atrial fibrillation (AF) [103]. More than 25% of patients with COPD will die as a result of CVD, and 40% of COPD patients with a cardiovascular history will die following a cardiovascular event [104]. There is an increased risk of CVD events, including ischemic heart disease, within the first 30 days after COPD exacerbations in patients with COPD with CVD [105]. It is well known that patients with comorbid COPD and CVD have worse outcomes than those with either condition alone [97]. The presence of CVD is associated with more severe breathlessness and a worse QOL, more frequent hospitalization, and higher mortality than those with COPD alone [104], while the presence of COPD is a predictor of hospitalization and death from cardiovascular events [106].
Despite established associations between COPD and CVD, it is usual for a patient’s presentations to be attributed to one disease only and for the other to be overlooked because of overlapping clinical symptoms and signs. In fact, in studies of COPD patients, excluding those with an existing CHF diagnosis, the prevalence of unrecognized CHF was about 20% [107,108]. Physical examinations such as peripheral edema, systolic murmur, and objective tests such as electrocardiography and the measurement of plasma brain natriuretic peptide can help physicians to identify concomitant CHF in COPD patients [108].
Adequate therapy for CVD is necessary without delay after a new diagnosis of CVD. However, pharmacotherapy for CVD is often withheld due to uncertain beliefs concerning safety for COPD patients. Even when CVD is known, patients with COPD receive less adequate CVD treatment than non-COPD patients [109,110]. In particular, β-blockers are essential medications in CHF, acute myocardial infarction, and AF. The pharmacologic properties of common β-blockers are summarized in Table 1 [111]. A meta-analysis of observational studies reported that β-blocker usage in COPD patients reduces the overall mortality and risk of COPD exacerbations (28% and 38%, respectively) [20]. Importantly, selective and non-selective β-blockers have different outcomes for patients with COPD. A previous randomized study that compared the effect of bisoprolol and carvedilol in patients with concurrent COPD and CHF confirmed that bisoprolol improved FEV1 and caused fewer adverse events than carvedilol [112]. In a retrospective study, patients taking bisoprolol were at a lower risk of CHF and/or COPD exacerbation than patients taking carvedilol [113]. In another cohort study, the rate of mortality and CHF exacerbations was lower in patients treated with bisoprolol compared to those with carvedilol or metoprolol [114]. Very recently, in a large-scale randomized controlled trial of metoprolol for patients with COPD that specifically excluded patients with established indications for a β-blocker, the metoprolol group did not show a lower risk of COPD exacerbations than the placebo group [115]. From the evidence so far, COPD patients without any cardiovascular comorbidity do not require beta-blockers, while the selective β-blocker bisoprolol should be used in COPD patients with CVD.
Table 1. Pharmacological properties of β-blockers.
|
|
Metoprolol |
Carvedilol |
Bisoprolol |
|
Daily oral dose * |
12.5–200 mg, QD |
3.125–100 mg, BID |
1.25–10 mg, QD |
|
Plasma t1/2 |
3–7 h |
6–10 h |
10–12 h |
|
Selectivity ratio (β1:β2) |
20:1 |
NA |
75:1 |
|
α-antagonism |
No |
Yes |
No |
|
Lipid solubility |
High |
Moderate |
Moderate |
|
Bioavailability |
50% |
30% |
>90% |
|
Clearance |
Liver |
Liver |
Liver/Kidney |
|
Metabolism |
CYP2D6 |
CYP2D6 |
CYP2D6 |
* Dosage for chronic heart failure. Abbreviations: QD, once a day; BID, twice a day; t1/2, half-time; NA, not available; CYP2D6, cytochrome P450 2D6.
Recently, the CLAIM study showed that dual bronchodilator treatment in hyperinflated patients with COPD reversed the detrimental lung-heart imbalance by increasing pulmonary microvascular blood flow, left ventricular end-diastolic volume, stroke volume, and regional ventilation [116,117]. These data clearly highlight the multidimensional benefits of maximum bronchodilation in patients with COPD.
References
- Pauwels, R.A.; Rabe, K.F. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004, 364, 613–620.
- Chapman, K.R.; Mannino, D.M.; Soriano, J.B.; Vermeire, P.A.; Buist, A.S.; Thun, M.J.; Connell, C.; Jemal, A.; Lee, T.A.; Miravitlles, M.; et al. Epidemiology and costs of chronic obstructive pulmonary disease. Respir. J. 2006, 27, 188–207.
- Global Initiative for Chronic Obstructive Lung Disease. 2020 Report: Global Strategy for Prevention, Diagnosis and Management of COPD. Available online: https://goldcopd.org/ gold -reports/ (accessed on 1 June, 2020).
- Donohue, J.F.; Jones, P.W.; Bartels, C.; Marvel, J.; D’Andrea, P.; Banerji, D.; Morris, D.G.; Patalano, F.; Fogel, R. Correlations between FEV1 and patient-reported outcomes: A pooled analysis of 23 clinical trials in patients with chronic obstructive pulmonary disease. Plum Ther. 2018, 49, 11–19.
- Han, M.K.; Agusti, A.; Calverly, P.M.; Celli, B.R.; Criner, G.; Curtis, J.L.; Fabbri, L.M.; Goldin, J.G.; Jones, P.W.; MacNee, W.; et al. Chronic obstructive pulmonary disease pnenotypes. The future of COPD. J. Respir. Crit. Care Med. 2010, 182, 598–604.
- Minakata, Y.; Morishita, Y.; Ichikawa, T.; Akamatsu, K.; Hirano, T.; Nakanishi, M.; Matsunaga, K.; Ichinose, M. Effects of pharmacologic treatment based on airflow limitation and breathlessness on daily physical activity in patients with chronic obstructive pulmonary disease. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1275–1282.
- Polverino, F.; Celli, B. The Challenge of controlling the COPD epidemic: Unmet needs. J. Med. 2018, 131, 1–6.
- Franssen, F.M.E.; Alter, P.; Bar, N.; Benedikter, B.J.; Iurato, S.; Maier, D.: Maxheim, M.; Roessler, F.K.; Spruit, M.A.; Vogelmeier, C.F.; et al. Personalized medicine for patients with COPD: Where are we? J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1465–1484.
- Matsunaga, K.; Oishi, K.; Miravitlles, M.; Anzueto, A. Time to revise COPD treatment algorithm. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2229–2234.
- Hanania, N.A.; O’Donnell, D.E. Activity-related dyspnea in chronic obstructive pulmonary disease: Physical and psychological consequences, unmet needs, and future directions. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1127–1138.
- Celli, B.R.; Wedzicha, J.A. Update on clinical aspects of chronic obstructive pulmonary disease. Engl. J. Med. 2019, 381, 1257–1266.
- Dransfield, M.T.; Kunisaki, K.M.; Strand, M.J.; Anzueto, A.; Bhatt, S.P.; Bowler, R.P.; Criner, G.J.; Curtis, J.L.; Hanania, N.A.; Nath, H. et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. J. Respir. Crit. Care Med. 2017, 195, 324-330.
- Anzueto, A.; Miravitlles, M. Chronic obstructive pulmonary disease exacerbations: A need for action. J. Med. 2018, 131, 15–22.
- Matsunaga, K.; Hayata, A.; Akamatsu, K.; Hirano, T.; Tamada, T.; Kamei, T.; Tsuda, T.; Nakamura, H.; Takahashi, T.; Hozawa, S. et al. Stratifying the risk of COPD exacerbation using the modified Medical Research Council scale: A multicenter cross-sectional CAP study. Investig. 2015, 53, 82–85.
- Oishi, K.; Hirano, T.; Hamada, K.; Uehara, S.; Suetake, R.; Yamaji, Y.; Ito, K.; Asami-Noyama, M.; Edakuni, N.; Matsunaga, K. Characteristics of 2017 GOLD COPD group A: A multicenter cross-sectional CAP study in Japan. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3901–3907.
- Akamatsu, K.; Matsunaga, K.; Sugiura, H.; Koarai, A.; Hirano, T.; Ichnose, M. Improvement of Airflow Limitation by Fluticasone Propionate/Salmeterol in Chronic Obstructive Pulmonary Disease: What is the Specific Marker? Front Pharmacol. 2011, 2, 6.
- Pavord, I.D.; Lettis, S.; Locantore, N.; Pascoe, S.; Jones, P.W.; Wedzicha, J.A.; Barnes, N.C. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax 2016, 71, 118–125.
- Yamaji, Y.; Oishi, K.; Hamada, K.; Ohteru, Y.; Chikumoto, A.; Murakawa, K.; Matsuda, K.; Suetake, R.; Murata, Y.; Ito, K. et al. Detection of type2 biomarkers for response in COPD. Breath Res. 2020, 14, 026007.
- Dransfield, M.T.; Rowe, S.M.; Johnson, E.; Bailey, W.C.; Gerald, L.B. Use of β blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax 2007, 63, 301–305.
- Du, Q.; Sun, Y.; Ding, N.; Lu, L.; Chen, Y. Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: A meta-analysis of observational studies. PLoS ONE 2014, 9, e113048.
- MacDonald, M.I.; Shafudain, E.; King, P.T.; Chang, C.L.; Bardin, P.G.; Hancox, R.J. Cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease. Lancet Respir. Med. 2016, 4, 138–148.
- Miravitlles, M.; Anzueto, A. Antibiotic prophylaxis in COPD: Why, when, and for whom? Pulm Pharmacol. Ther. 2015, 32, 119–123.
- Segal, L.N.; Clemente, J.C.; Wu, B.G.; Wikoff, W.R.; Gao, Z.; Li, Y.; Ko, J.P.; Rom, W.N.; Blaser, M.J.; Weiden, M.D. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax 2017, 72, 13–22.
- Lopez-Campos, J.L.; Miravitlles, M.; de la Rosa Carrillo, D.; Canton, R.; Soler-Cataluna, J.J.; Martinez-Garcia, M.A. Current challenges in chronic bronchial infection in patients with chronic obstructive pulmonary disease. J. Clin. Med. 2020, 28, e1639.
This entry is adapted from the peer-reviewed paper 10.3390/jcm9103078

