Leucine-rich repeats containing G protein-coupled receptor 4 (LGR4) is a receptor that belongs to the superfamily of G protein-coupled receptors that can be activated by R-spondins (RSPOs), Norrin, circLGR4, and the ligand of the receptor activator of nuclear factor kappa-B (RANKL) ligands to regulate signaling pathways in normal and pathological processes.
- LGR4
- GPR48
- cancer
1. Introduction
2. LGR4 Characterization
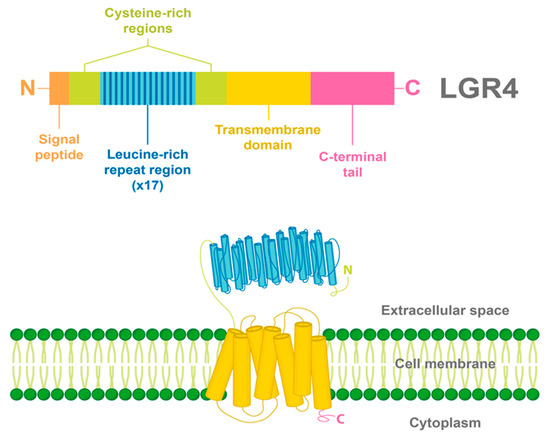
3. Ligands and Canonical Signaling Pathways Regulated by LGR4
3.1. R-Spondins (RSPOs)
LGR4 was considered an orphan receptor until 2011, when Carmon et al., Glynca et al., and Ruffner et al. performed co-immunoprecipitation, co-immunofluorescence, and binding assays to show that RSPOs bind LGR4 and modulate the Wnt signaling pathway [6][7][11].
R-spondins (roof plate-specific spondin) are a family of four secreted proteins (RSPOs 1-4) that were characterized for the first time in mice. These proteins share about 40–60% of amino acid identity between them and also have homologous structures [16]. Structurally, RSPOs have (1) a signal peptide at the N-terminus for secretion; (2) two furin-like cysteine-rich domains that are necessary for ligand activity; (3) a thrombospondin 1 repeat domain (TSR); and (4) a basic amino acid-rich domain with different lengths at the C-terminus [16][17][18] (Figure 2a). RSPOs can activate three of the eight members of the LGR’s family (LGR4/5/6) and can induce a potent and sustained activation of the Wnt pathway, a well-known signaling cascade involved in several physiological and pathological processes including cancer [6][7][11][19].
The Wnt signaling pathway is mainly activated by the frizzled/LRP5-6 receptor complex. LGR4, through its extracellular domain, can interact with any of the four RSPOs and enhance the Wnt signaling pathway. Stimulation of LGR4 by RSPOs stabilizes the Frizzled/Lrp5-6 complex in the membrane, avoiding its degradation by inhibiting the activity of ZNRF3 and RNF43 proteins [20][21][22][23] . Furthermore, LGR4 recruits IQGAP1, a scaffold protein that induces the recruitment of the β-catenin destruction complex to the Fzd/LRP5-6 receptor complex [24][25]. The interaction of the Frizzled/LRP5-6 receptor complex with the Wnt ligands results in the cytoplasmic accumulation of β-catenin and its later nuclear translocation. In the nucleus, β-catenin, in a complex with the TCF transcription factor, acts as a transcription regulator of its target genes (Figure 2b) [26][27]. Interestingly, RSPOs can also enhance Wnt signaling independently of LGR receptors [16][17].
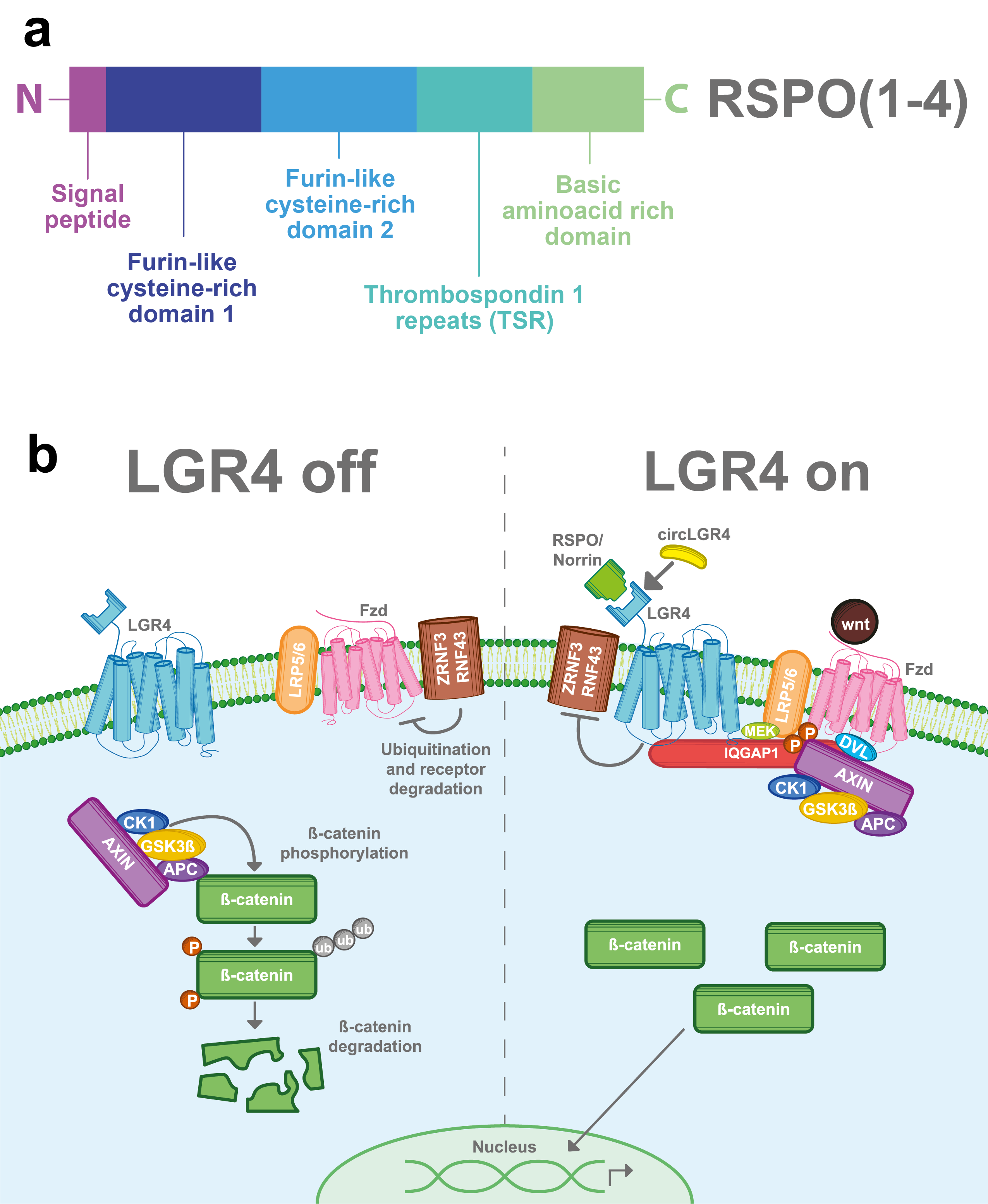
Figure 2. LGR4-induced Wnt/β-catenin signaling pathway. (a) RSPOs are a family of secreted proteins that can activate LGR4-induced Wnt/β-catenin signaling. Structurally, all the RSPOs have a signal peptide in the N-terminal domain, two furin-like cysteine-rich domains, a thrombospondin 1 repeat domain (TSR), and a basic amino acid-rich domain, which varies in size according to the RSPO member (b) In the absence of RSPO, ZNF3/RNF43 ubiquitinates the frizzled (Fzd)/LRP5-6 receptor complex for degradation. Wnt signal is blocked and the β-catenin destruction complex (formed by CK1, GSK3β, APC, and AXIN) is activated. GSK3β and CK1 phosphorylate β-catenin, inducing its ubiquitination and consequent proteasomal degradation. When LGR4 is activated by RSPOs, Norrin, or circLGR4 ligands, it stabilizes the frizzled/Lrp5-6 complex in the membrane, avoiding its degradation by inhibiting the activity of ZNRF3 and RNF43 proteins. Furthermore, LGR4 recruits IQGAP1 with an increasing affinity for DVL and recruits MEK, which phosphorylates LRP5/6, leading to the recruitment and inhibition of the β-catenin destruction complex into the Fzd/Lrp5-6 complex receptor.
3.2. Norrin
In 2013, Deng et al. found a second ligand for LGR4 [8]. Norrin is a secreted protein that can promote Wnt signaling and regulate physiological processes. Structurally, Norrin has an N-terminal signal peptide and a cysteine-rich C-terminal domain that allows its homodimerization. Similar to RSPOs, Norrin can also interact with LGR4, stabilize the frizzled/LRP5-6 receptor complex and thus enhance the Wnt/β-catenin signal (Figure 2b). In addition, Norrin can also interact with frizzled receptors and intensify the Wnt pathway in an LGR4-independent way [8][28].
3.3. RANKL
The ligand of the receptor activator of nuclear factor kappa-B (RANKL) is a homotrimeric type II membrane protein with no signal peptide, which belongs to the tumor necrosis factor (TNF) family of cytokines [29]. Three alternative spliced isoforms of RANKL have been detected; the full-length RANKL (RANKL1), a shorter form that lacks part of the cytoplasmic domain (RANKL2), and a soluble form with the N-terminal part deleted (RANKL3)[30]. RANKL was first characterized as a ligand of the RANK receptor, a well-known activator of the NF-κB signaling pathway, and both RANK and RANKL have been associated with bone remodeling by regulating osteoclast differentiation [29].
Since LGR4 has a typical GPCR structure, efforts have been made to search its G-protein-dependent function. In 2016, Luo et al. showed for the first time that RANKL binds to the extracellular domain of LGR4 and activates a Gαq protein that activates the GSK3β signaling pathway, thus suppressing the expression of the NFATC1, a key transcription factor for osteoclastogenesis [9].
It has been shown, in an osteoclast model, that LGR4 competes with RANK to bind RANKL and suppresses canonical RANK signaling, thus exerting an opposing effect on the RANK pathway. Interestingly, LGR4 is a downstream target of RANKL–RANK signaling, suggesting that LGR4 acts as a feedback loop controlling RANKL activities [9]. Furthermore, it has been suggested that RANKL can compete with RSPOs to bind LGR4 and, in this way, the interaction of RANKL/LGR4 can disrupt the Wnt/β-catenin signaling potentiated by RSPOs [9].
3.3. CircLGR4
CircLGR4 is a circular RNA (circ-LGR4) that encodes a 19 amino acid peptide, which is secreted through the Golgi pathway. Recently, in a colorectal cancer model Zhi, et al. showed that the circLGR4 peptide interacts with the extracellular domain of LGR4 and enhances Wnt/ β-catenin signaling (Figure 2b). Disruption of circLgr4 expression resulted in impaired colon cancer stem cell self-renewal, tumorigenesis, and invasion [10].
4. LGR4 in Cancer
4.1. Breast Cancer
4.2. Colorectal Cancer
4.3. Lung Cancer
4.4. Oral Cancer
4.5. Prostate Cancer
4.6. Skin Cancer
4.7. Other Cancers
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094690
References
- Weinberg, R.A. The Biology of Cancer, 2nd ed.; Garland Science, Taylor and Francis Group: New York and London, 2013, ISBN 9780429258794.
- Krauss, G. Biochemistry of Signal Transduction and Regulation; Wiley: Hoboken, NJ, USA, 2003; ISBN 9783527601868.
- Bar-Shavit, R.; Maoz, M.; Kancharla, A.; Nag, J.K.; Agranovich, D.; Grisaru-Granovsky, S.; Uziely, B. G protein-coupled receptors in cancer. Int. J. Mol. Sci. 2016, 17, 1–16, doi:10.3390/ijms17081320.
- Van Loy, T.; Vandersmissen, H.P.; Van Hiel, M.B.; Poels, J.; Verlinden, H.; Badisco, L.; Vassart, G.; Vanden Broeck, J. Comparative genomics of leucine-rich repeats containing G protein-coupled receptors and their ligands. Gen. Comp. Endocrinol. 2008, 155, 14–21, doi:10.1016/j.ygcen.2007.06.022.
- Petrie, E.J.; Lagaida, S.; Sethi, A.; Bathgate, R.A.D.; Gooley, P.R. In a class of their own-RXFP1 and RXFP2 are unique members of the LGR family. Front. Endocrinol. 2015, 6, 137, doi:10.3389/fendo.2015.00137.
- Carmon, K.S.; Gong, X.; Lin, Q.; Thomas, A.; Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 11452–11457, doi:10.1073/pnas.1106083108.
- Ruffner, H.; Sprunger, J.; Charlat, O.; Leighton-Davies, J.; Grosshans, B.; Salathe, A.; Zietzling, S.; Beck, V.; Therier, M.; Isken, A.; et al. R-spondin potentiates Wnt/β-Catenin signaling through orphan receptors LGR4 and LGR5. PLoS ONE 2012, 7, doi:10.1371/journal.pone.0040976.
- Deng, C.; Reddy, P.; Cheng, Y.; Luo, C.-W.; Hsiao, C.-L.; Hsueh, A.J.W. Multi-functional norrin is a ligand for the LGR4 receptor. J. Cell Sci. 2013, 126, 2060–2068, doi:10.1242/jcs.123471.
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C.; et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546, doi:10.1038/nm.4076.
- Zhi, X.; Zhang, J.; Cheng, Z.; Bian, L.; Qin, J. circLgr4 drives colorectal tumorigenesis and invasion through Lgr4‐targeting peptide. Int. J. Cancer 2019, doi:10.1002/ijc.32549.
- Glinka, A.; Dolde, C.; Kirsch, N.; Huang, Y.L.; Kazanskaya, O.; Ingelfinger, D.; Boutros, M.; Cruciat, C.M.; Niehrs, C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 2011, 12, 1055–1061, doi:10.1038/embor.2011.175.
- Hsu, S.Y.; Liang, S.-G.; Hsueh, A.J.W. Characterization of Two LGR Genes Homologous to Gonadotropin and Thyrotropin Receptors with Extracellular Leucine-Rich Repeats and a G Protein-Coupled, Seven-Transmembrane Region. Mol. Endocrinol. 1998, 12, 1830–1845, doi:10.1210/mend.12.12.0211.
- Loh, E.D.; Broussard, S.R.; Liu, Q.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Kolakowski, L.F., Jr. Chromosomal localization of GPR48, a novel glycoprotein hormone receptor like GPCR, in human and mouse with radiation hybrid and interspecific backcross mapping. Cytogenet. Genome Res. 2000, 89, 2–5, doi:10.1159/000015576.
- Loh, E.D.; Broussard, S.R.; Kolakowski, L.F. Molecular characterization of a novel glycoprotein hormone G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2001, 282, 757–764, doi:10.1006/bbrc.2001.4625.
- Yi, J.; Xiong, W.; Gong, X.; Bellister, S.; Ellis, L.M.; Liu, Q. Analysis of LGR4 Receptor Distribution in Human and Mouse Tissues. PLoS ONE 2013, 8, 1–11, doi:10.1371/journal.pone.0078144.
- Jin, Y.R.; Yoon, J.K. The R-spondin family of proteins: Emerging regulators of WNT signaling. Int. J. Biochem. Cell Biol. 2012, 100, 130–134, doi:10.1016/j.pestbp.2011.02.012.Investigations.
- Raslan, A.A.; Yoon, J.K. R-spondins: Multi-mode WNT signaling regulators in adult stem cells. Int. J. Biochem. Cell Biol. 2019, 106, 26–34, doi:10.1016/j.biocel.2018.11.005.
- Wang, D.; Huang, B.; Zhang, S.; Yu, X.; Wu, W.; Wang, X. Structural basis for R-spondin receptors. Genes Dev. 2013, 1339–1344, doi:10.1101/gad.219360.113.RSPO1.
- De Lau, W.; Barker, N.; Low, T.Y.; Koo, B.; Li, V.S.W.; Teunissen, H.; Kujala, P.; Haegebarth, A.; Peters, P.J.; Van De Wetering, M.; et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011, 476, 293–297, doi:10.1038/nature10337.
- Hao, H.X.; Xie, Y.; Zhang, Y.; Zhang, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–202, doi:10.1038/nature11019.
- Peng, W.C.; De Lau, W.; Madoori, P.K.; Forneris, F.; Granneman, J.C.M.; Clevers, H.; Gros, P. Structures of Wnt-Antagonist ZNRF3 and Its Complex with R-Spondin 1 and Implications for Signaling. PLoS ONE 2013, 8, 1–10, doi:10.1371/journal.pone.0083110.
- Xie, Y.; Zamponi, R.; Charlat, O.; Ramones, M.; Swalley, S.; Jiang, X.; Rivera, D.; Tschantz, W.; Lu, B.; Quinn, L.; et al. Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R-spondin. EMBO Rep. 2013, 14, 1120–1126, doi:10.1038/embor.2013.167.
- Moad, H.E.; Pioszak, A.A. Reconstitution of R-spondin:LGR4:ZNRF3 adult stem cell growth factor signaling complexes with recombinant proteins produced in escherichia coli. Biochemistry 2013, 52, 7295–7304, doi:10.1021/bi401090h.
- Li, Z.; Zhang, W.; Mulholland, M.W. LGR4 and Its Role in Intestinal Protection and Energy Metabolism. Front. Endocrinol. 2015, 6, 1–9, doi:10.3389/fendo.2015.00131.
- Carmon, K.S.; Gong, X.; Yi, J.; Thomas, A.; Liu, Q. RSPO-LGR4 functions via IQGAP1 to potentiate Wnt signaling. Proc. Natl. Acad. Sci. USA 2014, 111, doi:10.1073/pnas.1323106111.
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75, doi:10.4161/org.4.2.5851.
- Duchartre, Y.; Kim, Y.-M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hematol. 2016, 99, 141–149, doi:10.1016/j.critrevonc.2015.12.005.
- Braunger, B.M.; Tamm, E.R. The Different Functions of Norrin. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 801, pp. 679–683. ISBN 978-1-4614-0630-3.
- Renema, N.; Navet, B.; Heymann, M.-F.; Lezot, F.; Heymann, D. RANK-RANKL signalling in cancer. Biosci. Rep. 2016, 36, e00366, doi:10.1042/BSR20160150.
- Ikeda, T.; Kasai, M.; Utsuyama, M.; Hirokawa, K. Determination of three isoforms of the receptor activator of nuclear factor-κB ligand and their differential expression in bone and thymus. Endocrinology 2001, 142, 1419–1426, doi:10.1210/endo.142.4.8070.
- Global Cancer Observatory Available online: https://gco.iarc.fr/ (accessed on 21 January 2021).
- Yue, Z.; Yuan, Z.; Zeng, L.; Wang, Y.; Lai, L.; Li, J.; Sun, P.; Xue, X.; Qi, J. LGR4 modulates breast cancer initiation, metastasis, and cancer stem cells. FASEB J. 2018, 32, 2422–2437, doi:10.1096/fj.201700897R.
- Zhu, Y.; Xu, L.; Chen, M.; Ma, H.; Lou, F. GPR48 Promotes Multiple Cancer Cell Proliferation via Activation of Wnt Signaling. Asian Pac. J. Cancer Prev. 2013, 14, 4775–4778, doi:10.7314/apjcp.2013.14.8.4775.
- Gao, Y.; Kitagawa, K.; Hiramatsu, Y.; Kikuchi, H.; Isobe, T.; Shimada, M.; Uchida, C.; Hattori, T.; Oda, T.; Nakayama, K.; et al. Up-regulation of GPR48 induced by down-regulation of p27Kip1 enhances carcinoma cell invasiveness and metastasis. Cancer Res. 2006, 66, 11623–11631, doi:10.1158/0008-5472.CAN-06-2629.
- Wu, J.; Xie, N.; Xie, K.; Zeng, J.; Cheng, L.; Lei, Y.; Liu, Y.; Song, L.; Dong, D.; Chen, Y.; et al. GPR48, a poor prognostic factor, promotes tumor metastasis and activates β -catenin/TCF signaling in colorectal cancer. Carcinogenesis 2013, 34, 2861–2869, doi:10.1093/carcin/bgt229.
- Gugger, M.; White, R.; Song, S.; Waser, B.; Cescato, R.; Rivière, P.; Reubi, J.C. GPR87 is an overexpressed G-protein coupled receptor in squamous cell carcinoma of the lung. Dis. Markers 2008, 24, 41–50, doi:10.1155/2008/857474.
- Gong, X.; Yi, J.; Carmon, K.S.; Crumbley, C.A.; Xiong, W.; Thomas, A.; Fan, X.; Guo, S.; An, Z.; Chang, J.T.; et al. Aberrant RSPO3-LGR4 signaling in Keap1-deficient lung adenocarcinomas promotes tumor aggressiveness. Oncogene 2015, 34, 4692–4701, doi:10.1038/onc.2014.417.
- Zhang, L.; Song, Y.; Ling, Z.; Li, Y.; Ren, X.; Yang, J.; Wang, Z.; Xia, J.; Zhang, W.; Cheng, B. R-spondin 2-LGR4 system regulates growth, migration and invasion, epithelial-mesenchymal transition and stem-like properties of tongue squamous cell carcinoma via Wnt/β-catenin signaling. EBioMedicine 2019, 44, 275–288, doi:10.1016/j.ebiom.2019.03.076.
- Al-Samadi, A.; Salo, T. Understanding the role of the R-spondin 2-LGR4 system in tongue squamous cell carcinoma progression. EBioMedicine 2019, 44, 8–9, doi:10.1016/j.ebiom.2019.05.033.
- Luo, W.; Tan, P.; Rodriguez, M.; He, L.; Tan, K.; Zeng, L.; Siwko, S.; Liu, M. Leucine-rich repeat– containing G protein– coupled receptor 4 (Lgr4) is necessary for prostate cancer metastasis via epithelial–mesenchymal transition. J. Biol. Chem. 2017, 292, 15525–15537, doi:10.1074/jbc.M116.771931.
- Liang, F.; Yue, J.; Wang, J. GPCR48/LGR4 promotes tumorigenesis of prostate cancer via PI3K/Akt signaling pathway. Med. Oncol. 2015, 32, doi:10.1007/s12032-015-0486-1.
- Zhang, J.; Li, Q.; Zhang, S.; Xu, Q.; Wang, T. Lgr4 promotes prostate tumorigenesis through the Jmjd2a/AR signaling pathway. Exp. Cell Res. 2016, 349, 77–84, doi:10.1016/j.yexcr.2016.09.023.
- Liang, F.; Zhang, H.; Cheng, D.; Gao, H.; Wang, J.; Yue, J.; Zhang, N.; Wang, J.; Wang, Z.; Zhao, B. Ablation of LGR4 signaling enhances radiation sensitivity of prostate cancer cells. Life Sci. 2021, 265, 118737, doi:10.1016/j.lfs.2020.118737.
- Hou, Q.; Han, S.; Yang, L.; Chen, S.; Chen, J.; Ma, N.; Wang, C.; Tang, J.; Chen, X.; Chen, F.; et al. The interplay of microRNA-34a, LGR4, EMT-associated factors, and MMP2 in regulating uveal melanoma cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4503–4510, doi:10.1167/iovs.18-26477.
- Xu, P.; Dang, Y.; Wang, L.; Liu, X.; Ren, X.; Gu, J.; Liu, M.; Dai, X.; Ye, X. Lgr4 is crucial for skin carcinogenesis by regulating MEK/ERK and Wnt/b -catenin signaling pathways. Cancer Lett. 2016, 383, 161–170, doi:10.1016/j.canlet.2016.09.005.
- Wang, Z.; Jin, C.; Li, H.; Li, C.; Hou, Q.; Liu, M.; Da, X.; Dong, E.; Tu, L. GPR48-Induced keratinocyte proliferation occurs through HB-EGF mediated EGFR transactivation. FEBS Lett. 2010, 584, 4057–4062, doi:10.1016/j.febslet.2010.08.028.
- Yu, C.; Liang, G.; Du, P.; Liu, Y. Lgr4 Promotes Glioma Cell Proliferation through Activation of Wnt Signaling. Asian Pac. J. Cancer Prev. 2013, 14, 4907–4911, doi:10.7314/apjcp.2013.14.8.4907.
- Kang, Y.E.; Kim, J.; Kim, K.S.; Chang, J.Y.; Lee, J.; Yi, S.; Kim, H.W.; Kim, J.T.; Lee, K.; Choi, M.J.; et al. Upregulation of RSPO2-GPR48/LGR4 signaling in papillary thyroid carcinoma contributes to tumor progression. Oncotarget 2017, 8, 114980–114994, doi:10.18632/oncotarget.22692.
- Zeng, Z.; Ji, N.; Yi, J.; Lv, J.; Yuan, J.; Lin, Z.; Liu, L.; Feng, X. LGR4 overexpression is associated with clinical parameters and poor prognosis of serous ovarian cancer. Cancer Biomarkers 2020, 28, 65–72, doi:10.3233/cbm-191145.
- Liu, J.; Wei, W.; Guo, C.A.; Han, N.; Pan, J.F.; Fei, T.; Yan, Z.Q. Stat3 upregulates leucine-rich repeat-containing G protein-coupled receptor 4 expression in osteosarcoma cells. Biomed Res. Int. 2013, 2013, doi:10.1155/2013/310691.
- Steffen, J.S.; Simon, E.; Warneke, V.; Balschun, K.; Ebert, M.; Röcken, C. LGR4 and LGR6 are differentially expressed and of putative tumor biological significance in gastric carcinoma. Virchows Arch. 2012, 461, 355–365, doi:10.1007/s00428-012-1292-1.
- Wang, Z.; Yin, P.; Sun, Y.; Na, L.; Gao, J.; Wang, W.; Zhao, C. LGR4 maintains HGSOC cell epithelial phenotype and stem-like traits. Gynecol. Oncol. 2020, 159, 839–849, doi:10.1016/j.ygyno.2020.09.020.
- Tan, B.; Shi, X.; Zhang, J.; Qin, J.; Zhang, N.; Ren, H.; Qian, M.; Siwko, S.; Carmon, K.; Liu, Q.; et al. Inhibition of RSPO-LGR4 facilitates checkpoint blockade therapy by switching macrophage polarization. Cancer Res. 2018, 78, 4929–4942, doi:10.1158/0008-5472.CAN-18-0152.

