First Generation of Prebiotics
Prebiotics were first described by Gibson and Roberfroid in 1995 as “non-digestible food ingredients that beneficially influence the health of the host by stimulating the activity of one or more commensal colon bacteria” [17]. The acquisition of new scientific data on their mode of action and their specificity allowed refining of this definition to being requalified in 2017, by the International Scientific Association of Probiotics and Prebiotics (ISAPP), as “substrates selectively used by micro-organisms of the host conferring benefits for his health”. Prebiotics must meet three criteria: 1: Be resistant to digestion in the stomach and upper intestine, 2: Be fermentable by the gut microbiota and 3: Specifically stimulate the growth and/or activity of intestinal bacteria beneficial to our health[18]. The benefits of prebiotics are not limited to the gut, they can also act systemically [19]. Indeed, new original studies demonstrate that prebiotics can also modulate IS and facilitate many biologic processes, including infection prevention and the improvement of mood and memory [19].
Prebiotics are usually composed of linked sugars such as oligosaccharides and short-chain polysaccharides (see Figure 1). These molecules have the chemical characteristic of being indigestible by the enzymes present in the intestinal tract. They will therefore be able to serve as nutritional substrates for microorganisms considered “beneficial” such as Bifidobacterium and Bacteroides or come into direct contact with the surrounding cells [20]. Fructans such as fructo-oligosaccharides (FOS) and inulin, as well as galactans such as galacto-oligosaccharides (GOS) are the most studied prebiotics for their modulatory effects on the microbiota. The main proven and assumed prebiotics are shown in Table 1 (from Afssa, 2005). The prebiotics that are most commonly found to occur naturally in our foods are the FOS and inulin. They are found in plant-based foods such as in some vegetables (leek, onion, garlic, artichoke, chicory and asparagus), fruits (banana) and cereals (rye, corn). With a balanced European diet, 3–11 g of natural prebiotics can be consumed per day [21]. In comparison, only 1–4 g is usually consumed daily in the USA. Prebiotics are also produced commercially as supplements through the hydrolysis of polysaccharides or enzymatic reactions from lower molecular weight sugars. Although there are many commercially available foods and food ingredients that claim to be prebiotics, currently only lactulose, FOS and GOS have a proven prebiotic effect and status.
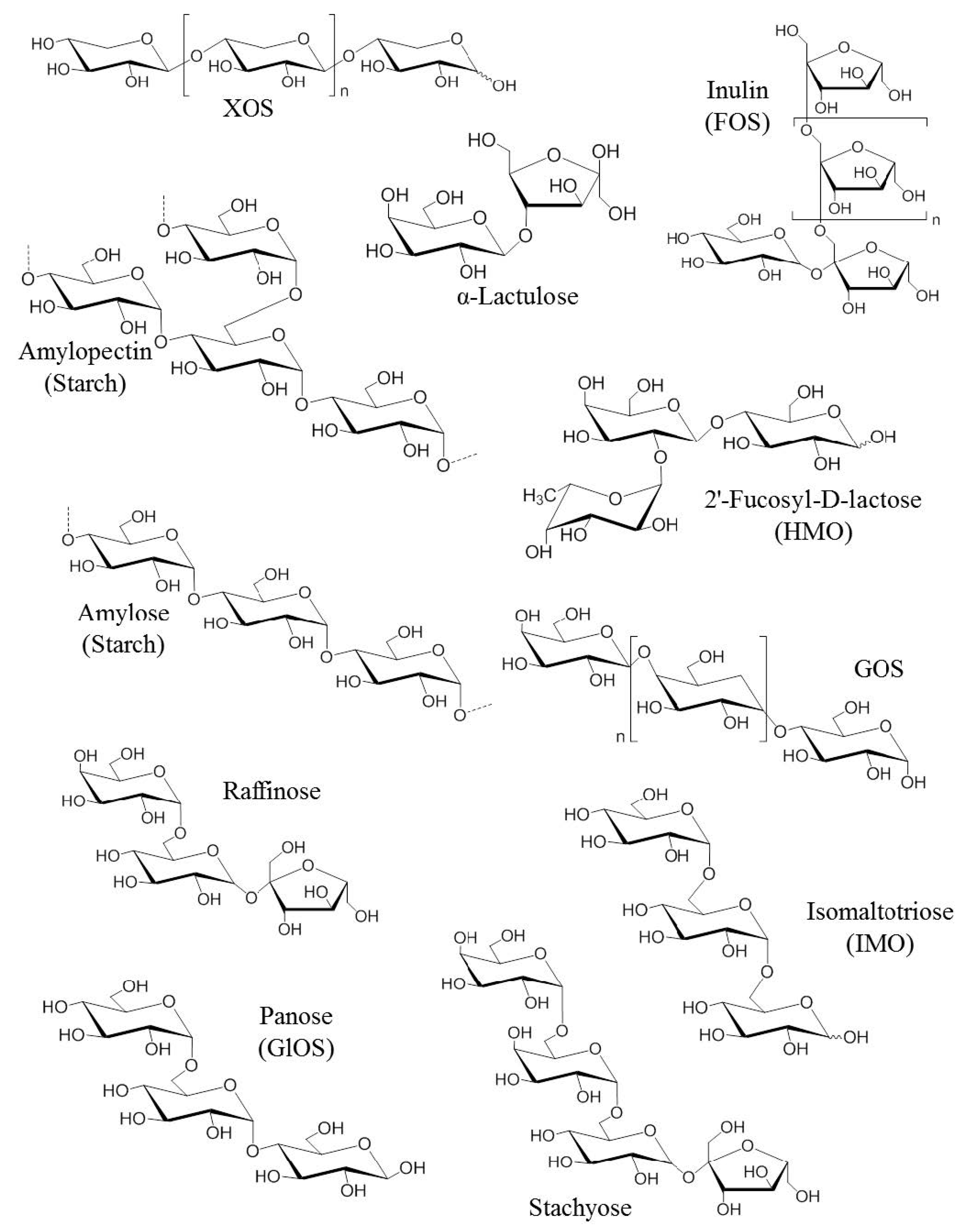
Figure 1. Chemical structures of the first generation of prebiotics. FOS: Fructo-oligosaccharides, GlOS: Gluco-oligosaccharides, GOS: Galacto-oligosaccharides, HMO: Human milk oligosaccharides, IMO: Isomalto-oligosaccharids [22].
Table 1. List of proven and assumed prebiotics.
|
Substance |
Composition |
Degree of polymerization |
Process of obtaining |
|
Fructans Linear Inulin Fructo-oligosides (FOS) et oligofructose (OF) Levans Connected (graminans) |
Glucose, fructose
2,1 bonds -2,1 bonds
-2,6 bonds
2,6 & 2,1 bonds |
10 to 60 2 to 9
20–30 (from vegetal)* unknown |
Extraction Synthesis, hydrolysis Enzymatic
Enzymatic biosynthesis |
|
Lactulose |
Galactose, fructose, 1,4 bonds |
2 |
Chimic synthesis |
|
(Trans)galacto-oligosides (TOS) |
Glucose, galactose, 1,6 bonds |
2 to 5 |
Enzymatic biosynthesis |
|
Galacto-oligosides (GOS) |
Glucose, galactose, 1,6 bonds |
2 to 5 |
Enzymatic biosynthesis |
|
Xylo-oligosides (XOS) |
Xylose, 1,4 bonds |
2 to 9 |
Enzymatic hydrolysis |
|
Soy oligosides or -galactosides (raffinose, stachyose & verbascose) |
Galactose, fructose, glucose, 1,6 and 1,2 bonds |
3 to 5 |
Extraction |
|
Isomaltooligosides |
Glucose, 1,6 bonds |
2 to 5 |
Enzymatic hydrolysis Enzymatic bioconversion |
|
Oligolaminarans (-glucans) |
Glucose (± mannitol), 1,3 and 1,6 bonds |
5 to 25 |
Enzymatic hydrolysis |
|
Polydextrose |
Poly-D-glucose (glucose 89%, sorbitol 10% and phosphoric acid 0.1%) |
12 (mean of DP) |
Chimic synthesis |
|
D-tagatose |
Tagatose |
1 |
Extraction |
|
Starch resistant |
Glucose, 1,4 and 1,6 bonds |
> 1000 |
Extraction |
In blood: Proven prebiotics. * Levans produced by microorganisms have usually molecular weights superior to 106.
Second Generation of Prebiotics
A second generation of prebiotics was designed in the 2000s to improve their functionality. The strategy consisted of adapting the chemical structure of prebiotics by a digestion with a specific enzyme produced by a probiotic. The prebiotic Bimuno (Clasado Biosciences Ltd.) is a good example. It corresponds to a GOS mixture produced from lactose using enzymes from the probiotic Bifidobacterium bifidum NCIMB 41171. It has:
- a highly selective and powerful prebiotic effect (growth of beneficial Bifidobacterium and improved colonization resistance) [23]. It has been demonstrated to be effective as a prebiotic in healthy adults, healthy elderly adults, irritable bowel syndrome (IBS) sufferers and overweight adults [24–26],an anti-invasive function (increasing protection against bacterial pathogens). It significantly reduces pathology and colonization associated with food-borne salmonellosis. It also reduces the incidence, severity and duration of traveller’s diarrhea [27–29].
- the ability to interact directly with the IS improving barrier function in the gut. Its positive immunomodulatory effect was further demonstrated in overweight adults with metabolic syndrome, where it significantly increased gut immune parameters involved in the protection against pathogens, as well as reducing blood and faecal inflammatory markers [25].
Human Milk Oligossacharides
Prebiotics are present in human milk and they are called human milk oligossacharides (HMOs). Human milk and colostrum are composed of oligosaccharides (5 g/L to 23 g/L) containing a lactose-reducing end elongated with fucosylated and/or sialylated N-acetyllactosamine units. There are over 150 HMOs structures that vary in their size, charge, and sequence [30]. The most frequent HMOs are the neutral fucosylated and non-fucosylated oligosaccharides [31]. The amount and structure of these HMOs can be really different between women and is dependent upon the Secretor and Lewis blood group status (see Figure 2). A deficiency of α 1, 2-linked fucosylated oligosaccharides in human milk is linked to the mutations in the fucosyltransferase 2 (FUT2) secretor gene. HMOs give no direct nutritional value to the infant, and are minor absorbed across the intestinal wall [32]. Instead, it is suggested, that HMOs can play many other roles for the infant. They are preferred substrates for several species of gut bacteria and act as prebiotics, promoting the growth of beneficial intestinal flora and shaping the gut microbiome. Short-chain fatty acids (SCFA) induced by the gut microbiome fermentation of HMOs are critical for intestinal health [33]. They stimulate the growth of gut commensal bacteria along with giving nourishment for epithelial cells lining the gut. HMOs also directly modulate host-epithelial responses, favoring reduced binding of pathogens to the gut barrier. Intestinal microbiota composition varies between formula-fed and breastfed infants, maybe due to the lack of HMOs in infant formula milk [34]. HMOs act probably as decoy receptors, inhibiting the binding of enteric pathogens to prevent infection and subsequent illness. Furthermore, HMOs induce a selective advantage for colonization by favorable bacteria, thereby inhibiting the growth of pathogenic species.
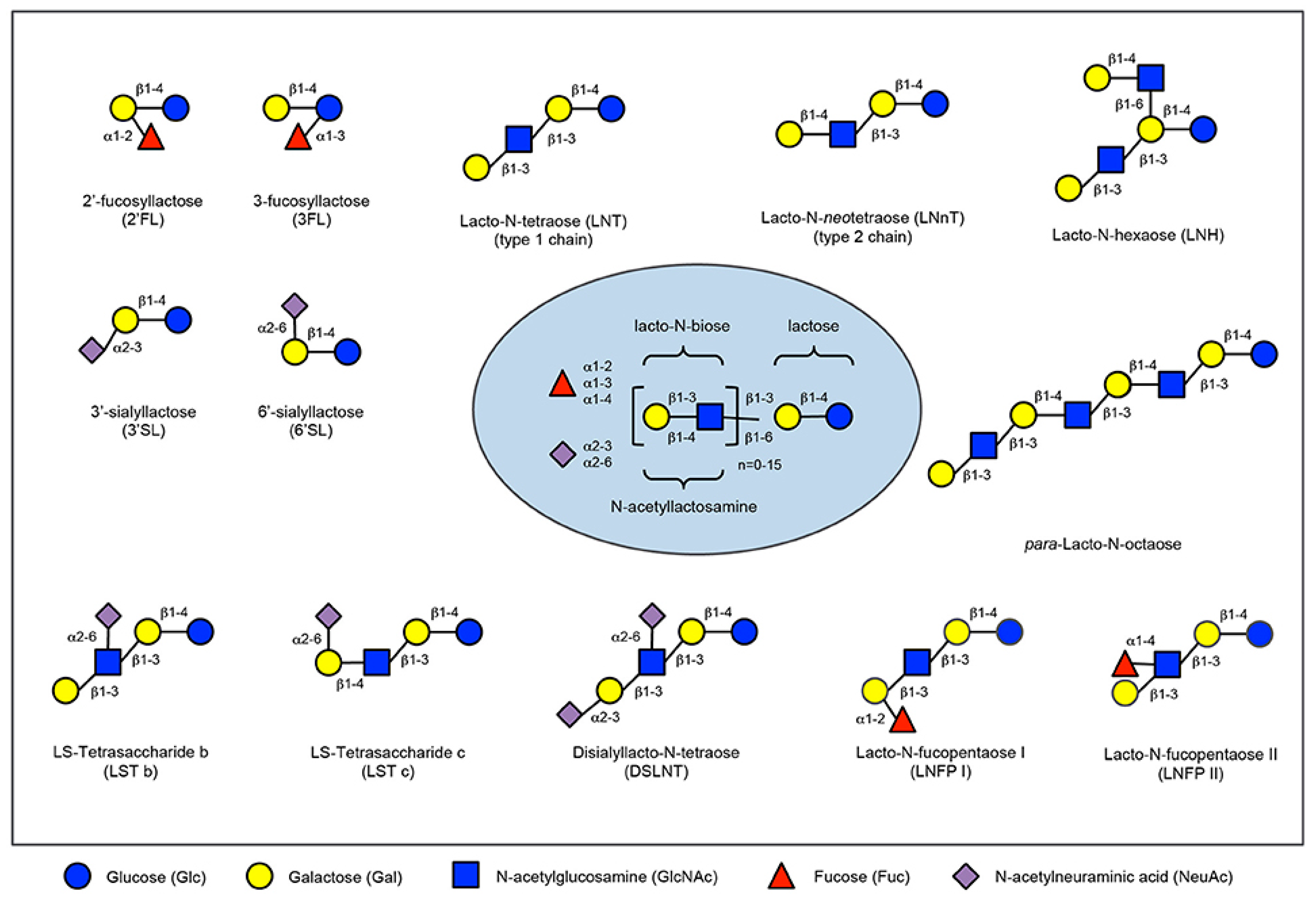
Figure 2. Human milk oligosaccharide composition blueprint.
The HMO composition follows a basic blueprint shown in the center. HMOs contain five different monosaccharides in different numbers and linkages, namely glucose (blue circle), galactose (yellow circle), N-acetlylactosamine (blue square), fucose (red triangle), and sialic acid (purple diamond). All HMOs carry lactose at the reducing end. Lactose can be fucosylated or sialylated to induce the small HMOs 2′-fucosyllactose and 3-fucosyllactose or 3′-sialyllactose and 6′-sialyllactose, respectively (upper left corner). Alternatively, lactose can be elongated with type 1 or type 2 disaccharide units to form linear or branched HMOs (upper right corner). Elongated HMOs then can be sialylated (lower left corner) or fucosylated (lower right corner) or both sialylated and fucosylated (not shown). The HMOs in this figure are only a few relatively simple examples. So far, more than 150 different HMO structures have been characterized [35].
Prebiotics Mechanisms
Prebiotics can influence the host health by two distinct mechanisms: indirect (see figure 3) or direct (see figure 4). Indirectly, the prebiotics acts as a fermentable substrate for some specific commensal host bacteria. This nutrient source allows the growth of specific taxa and lead to a modulation of gut intestinal microbiota. The SCFAs released in the gut intestinal tract influence many molecular and cellular processes. Recently, new research explored the direct effect of prebiotics on several compartments and specifically on different patterns of cells (epithelial and immune cells).
Indirect Effects: The Short Chain Fatty Acids (see Figure 3)
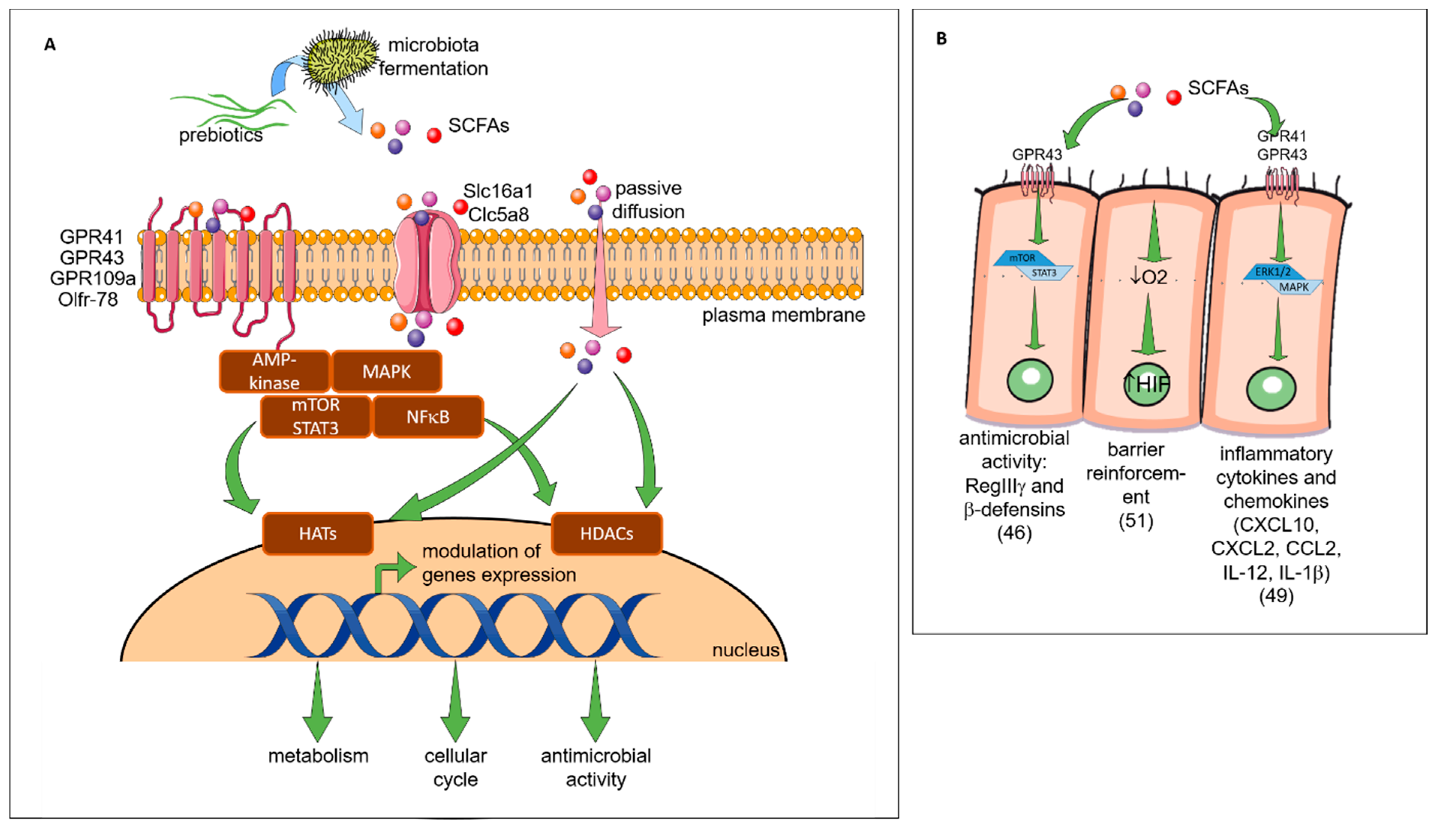
Figure 3. Indirect effects of prebiotics. (A) The general mechanisms of SCFAs. The SCFAs are metabolites derived from the fermentation of prebiotics by the microbiota. They are consumed by the microbiota or released into the biological systems (blood, gut, lung, placenta). They can interact with the cells by three mechanisms. In the first mechanism, the GPRs which are receptors coupled to signaling pathways (AMP-activated protein kinase (AMP-K), mammalian target of rapamycin (mTOR), signal transducer and activator of transcription 3 (STAT3), mitogen-activated protein kinases (MAPKs), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)) are involved. The second mechanisms correspond to the diffusion channels (solute carrier family 16 member 1 (Slc) 16a1 and 5a8) that allow the SCFAs transport directly to cytoplasm and their potential interactions with pathways. By these two mechanisms, the signaling cascade is activated and can influence the transcription of genes by acetylation and deacetylation respectively via the histone acetyltransferases (HAT) and the histone deacetylases (HDAC) enzymes (epigenetic mechanisms). In the last mechanisms, there is a passive diffusion of SCFAs able to modulate directly enzymes (HDAC, HAT) involved in epigenetic processes. The modulation of genes expression by acetylation and deacetylation will have different consequences such as a modification of metabolism, cell cycle or microbial activity described in Figure 3B,C. (B) The specific impact of SCFAs on epithelial cells. SCFAs (butyrate, propionate) can interact with the G-protein coupled receptor (GPR)43 receptor and activates the mTOR/STAT3 pathway allowing the modulation of genes to increase the expression of antimicrobial peptides such as regenerating islet-derived protein 3 gamma (RegIIIγ) and β-defensins. SCFAs can directly increase the epithelial barrier function by stimulating O2 metabolism in intestinal epithelial cell lines. This mechanism results in the stabilization of the transcription factor hypoxia-inducible factor (HIF-1). SCFAs interact also with the GPR 41 (acetate, propionate) and GPR43 receptors to activate the extracellular signal-regulated kinases (ERK) 1/2 and MAPK signaling pathway. In this way, epithelial cells produce inflammatory chemokines and cytokines during the immune response to protect the organism against aggressions or infections. The consumption of SCFA also increases the secretion of the antimicrobial peptide by epithelial cells. (C) The specific impact of SCFAs on IS. SCFAs can be found in the bloodstream. They can interact with different immune cell subtypes. In a first step they can modify the hematopoeisis of dendritic cells (DC) precursors in the bone marrow and induce CD11c+ CD11b+ DCs in lung-draining lymph nodes. The cells CD11c+ CD11b+ DC have a lower capacity to activate the Th2 cell which results in the reduction of allergic asthma. SCFAs are also able to modify in vitro the functionality of FMS-like tyrosine kinase 3 ligand (Flt3L)-elicited splenic DCs: a lower ability to activate T cell and to transport antigen to the lymph node and a lower expression of chemokine (C-C motif) ligand 19 (CCL19) on their surface decreasing their ability to move in different sites. In the lungs SCFAs are able to inhibit the enzyme HDAC9 resulting in an increase of the forkhead box P3 (FoxP3) transcription factor and then an increase of the number and the activity of Treg. In the intestine the increase of retinoic acid-synthesizing (RALDH) enzyme activity during the consumption of SCFA, allows the conversion of vitamin A to the retinoic acid in tolerogenic DC CD103 +. Then, the retinoic acid acts directly on T cells and induces their differentiation into Treg.
Prebiotics are highly fermentable food ingredients. This feature promotes expansion and stimulates the implantation of some beneficial and bifidogenic bacteria. Indeed, it was shown that inulin consumption increases specifically and significantly the abundances of Bifidobacteria and Lactobacilli [17,36,37]. These gut microbiota modifications improve the host health, notably by inhibiting pathogen implantation in the gut. The increase of Bifidobacteria following prebiotic consumption was correlated with an increase of acetate production by Bifidobacteria, a decrease of the C. difficile pathogen population in the gut intestinal tract and an inhibition of pathogens translocation from the gut lumen to the blood [38,39]. The benefits of Bifidobacteria are well described in the literature, recent studies reported that new prebiotics (apple pectin and 1-kestose) efficiently stimulate the proliferation of Faecalibacterium prausnitzii known to possess an anti-inflammatory effect [40]. Indeed, the pectin promotes the expansion of Faecalibacterium prausnitzii, but also Eubacterium eligens DSM3376, which strongly improves in vitro the secretion of the anti-inflammatory cytokine IL-10 [41].
Prebiotics, by their ability to be fermented by bacteria, induce the production of SCFAs and thus act indirectly on health. SCFAs can be used in the gut intestinal tract by the microbiota for their own metabolism or released in the lumen. In the lumen, SCFAs can specifically interact with different cells such as intestinal epithelial cells (IEC) or innate/adaptive immune cells to modify various cellular processes as well as gene expression, differentiation, proliferation and apoptosis. SCFAs can activate G protein coupled receptors (GPRs), such as GPR41 or the free fatty acid receptor (Ffar)3 (acetate = propionate > butyrate), GPR43 or Ffar2 (butyrate = propionate > acetate), GPR109a (butyrate) and olfactory receptor (Olfr)-78 (propionate = acetate), to modulate cell development, function and survival [42]. They can also enter directly into the cells through transporters solute carrier family 16 member 1 (Slc) 16a1 and Slc5a8 or by passive diffusion to subsequently induce signaling pathways [43,44]. Signaling pathway induced by SCFAs-binding are mediated by various actors: protein kinases, such as the AMP-activated protein kinase (AMPK) [45], mitogen-activated protein kinases (MAPK) [46], mammalian target of rapamycin (mTOR), signal transducer and activator of transcription 3 (STAT3) [47] or nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) [48]. Either by downstream signaling pathways or directly, SCFAs modulate the function of several enzymes and transcription factors including histone acetyltransferase (HATs) or histone deacetylases (HDACs) [49]. Consequences of SCFAs-related genes transcription modification is well described: modification of cellular cycle, anti-microbial effects and metabolism (regulation of lipogenesis and lipolysis in the cytosol of hepatocytes and adipocytes, and action on central appetite regulation) [42]. In the next section of this review, we focus on pro- and anti-inflammatory effects induced by SCFAs on epithelial and immune cells.
Epithelial Cells
The SCFAs interact directly with IEC in the intestinal tract. It was demonstrated that this interaction can influence the intestinal protective immunity via IEC cytokines secretion by Kim et al. who have shown that SCFAs activate GPR41 and GPR43 on IEC, promoting an inflammatory response [50]. Notably, the production of chemokines (C-X-C Motif Chemokine Ligand (CXCL)10, CXCL2, and Chemokine (C-C motif) ligand (CCL)2) and cytokines (IL-12, IL-1) was induced. On the other hand, GPRs-dependent activation of SCFAs is implicated in the regulation of antimicrobial peptide expression on IEC activating mTOR and STAT3 signaling. These mechanisms protect the host by preventing the pathogenesis implantation in the gut [47]. By modulating the activity of IEC, SCFAs can indirectly interact with immune cells such as dendritic cells (DCs). It has been shown that SCFAs increase the vitamin-A converting enzyme in IEC leading to a raised number of intestinal tolerogenic DCs and Treg [51]. SCFAs also promote the gut barrier function itself inducing the production of IL-18, a cytokine that contributes to the intestinal epithelium homeostasis [52,53]. SCFAs can also be found in peripheral blood where they can potentially act on other epithelial cells [54]. Indeed, Qian et al. have shown that feeding asthmatic mice with SCFAs (butyric acid) could reduce the damage of the lung epithelial barrier in asthmatic patients and decrease airway inflammation[55].
Immune Cells
The SCFAs-derived from microbiota fermentation can directly influence the phenotype or/and activity of various innate and adaptive immune cells. [42]. Some studies reported the implication of SCFAs in allergy protection. Trompette et al. have shown that asthmatic mice which received a high-fiber diet had a significant increase of blood SCFAs levels and were protected against the allergic airway disease [56]. They demonstrated that circulating SCFAs can go to the bone marrow to alter hematopoiesis characterized by enhanced generation of macrophage and DC precursors and subsequent seeding of the lungs by DCs with high phagocytic capacity but an impaired ability to promote the Th2 cell function. They conclude that SCFAs can shape the immunological environment in the lung and influence the severity of allergic inflammation.. Cait et al. also investigated the mechanism of SCFAs to decrease the allergic airway inflammation and demonstrated that DCs in contact with SCFAs are less able to stimulate T cells, to migrate in response to CCL19 in vitro, and to transport inhaled allergens to lung draining nodes [57]. Thorburn et al., demonstrated that SCFAs enter the bloodstream and inhibit HDACs, leading to the transcription of forkhead box P3 (Foxp3) [58]. Foxp3 stimulates the number and the function of Treg, which suppresses the airway inflammation. Additionally in their study, adult offspring (after prebiotics were fed to pregnant mice) were unable to develop the airway disease. These effects of prebiotic supplementation were mediated in utero independently of microbial transfer as SCFAs are able to cross the placenta. Once in the fetus, SCFAs influence gene expression in the fetal lung, such as natriuretic peptide A ,this encodes the atrial natriuretic peptide, a molecule implicated in the modulation of epithelial physiology and IS[58].
In the context of FA, Tan et al. have shown that a high-fiber dietin mice promoted oral tolerance and protected from FA via SCFAs signaling [59]. The molecular mechanism highlighted leading to food tolerance was the enhancement of the retinal dehydrogenase activity in CD103+DC, promoting differentiation of Treg cells. This specific diet also increased the IgA production and enhanced the T follicular helper and mucosal germinal center responses.
In conclusion, prebiotics can indirectly effect the gut and the IS by SCFA production. By its regulatory effect, SCFAs can potentially influence and prevent different diseases such as allergies. Nevertheless, the effect of SCFAs and modification of microbiota are not the only mechanism of prebiotics.
Direct Effect of Prebiotics (see Figure 4)
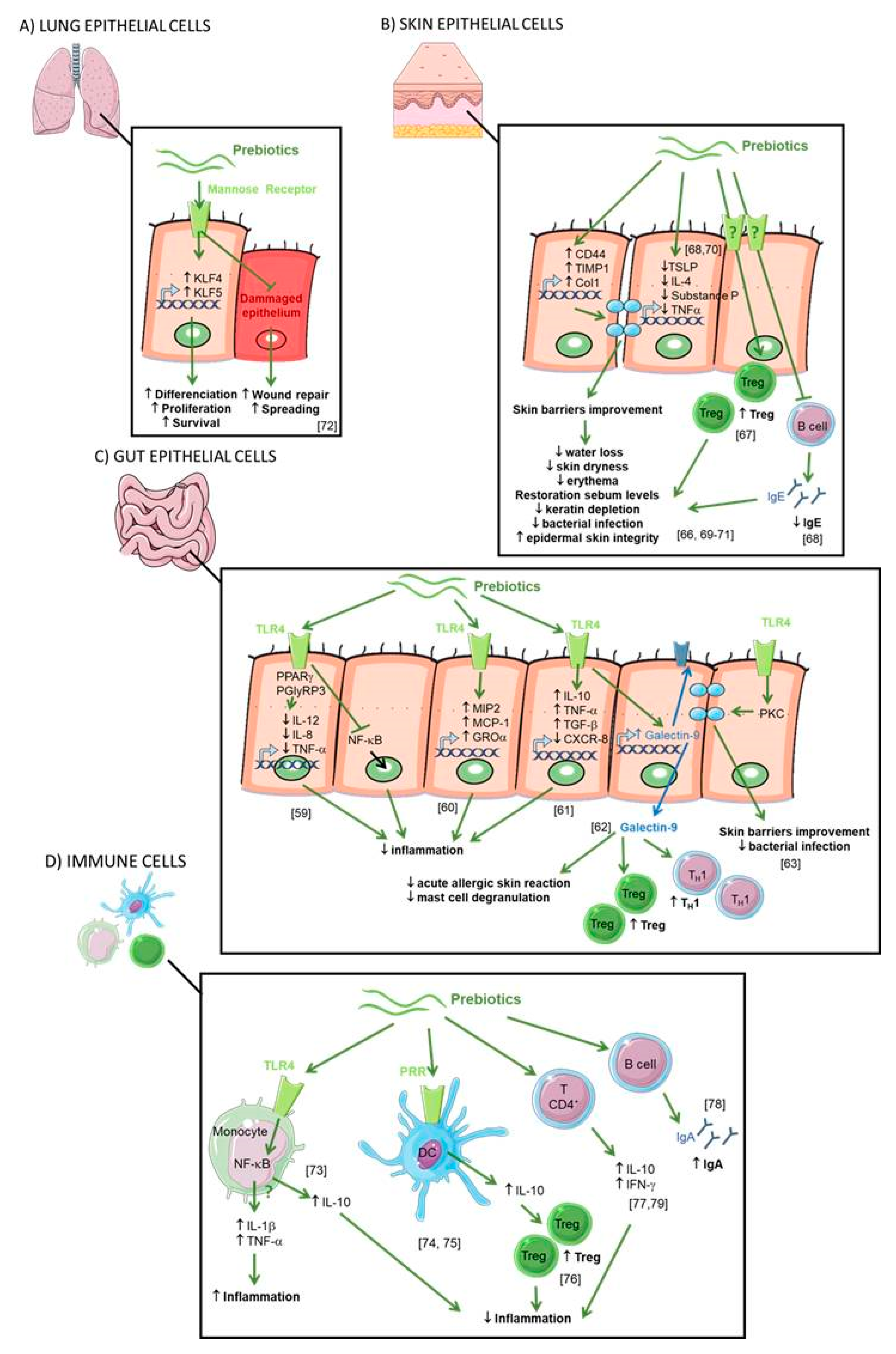
Figure 4. Direct effects of prebiotics. (A) Direct effect of prebiotics on lung epithelial cells. Mannan prebiotic stimulates cell spreading and facilitates wound repair in damaged human bronchial epithelium, involving mannose receptors. Prebiotics also increases expression and activation of Krüppel-like factors (KLFs) inducing cell differentiation, survival, and proliferation. (B) Direct effect of prebiotics on skin epithelial cells. Prebiotics supplementation improved water retention and prevented erythema via the expression of CD44, metallopeptidase inhibitor 1 (TIMP)-1, and collagen type 1(Col1) improving the skin’s barrier properties. Prebiotics suppress overproductions of thymic stromal lymphopoietin (TSLP), substance P, IL-10, IL-4, and tumor necrosis factor (TNF)-a leading to reduced transepidermal water loss and skin dryness, prevention of keratin depletion, improvement of biophysical parameters of the epidermis, restoration of skin sebum levels, and limitation of bacterial infection. Prebiotics increase CD4+ Foxp3+ Treg in skin lymph nodes and prevent germline class-switching and IgE production. (C) Direct effect of prebiotics on gut epithelial cells. Prebiotics are Toll like receptor 4 (TLR4) ligands in IEC. Prebiotics induce a range of anti-inflammatory cytokines and reduce pro-inflammatory cytokines to inhibit gut inflammation. Prebiotics enhance galectin-9 expression correlated with reduced acute allergic skin reaction and mast cell degranulation and promoted Th1 and Treg responses. Prebiotics directly promoted barrier integrity to prevent pathogen-induced barrier disruptions involving the induction of protein kinase C (PKC). (D) Direct effect of prebiotics on immune cells. Prebiotics induce both the secretion of anti-inflammatory (IL-10) and pro-inflammatory (IL-1β and TNF-α) cytokines by blood monocytes due to the activation of the NF-ĸB pathway by the binding of TLR4. Prebiotics bind pathogen recognition receptor (PRRs) on the surface of DCs inducing IL-10 secretion and Treg cells. Prebiotics enhance the secretion of IL-10 and interferon-γ (IFN-γ) by CD4+ T cells and IgA.
IEC
It is well described that prebiotics modulate the gut microbiota leading to the decrease of intestinal inflammation. However, some studies suggest that oligosaccharides may exert an anti-inflammatory effect per se, also called “non-prebiotic effects”. When ingested, prebiotics enter the intestine and are in direct contact with the gut epithelial cells. Zenhom et al., have demonstrated in vitro the anti-inflammatory effect of prebiotics on enterocyte lineages, characterized by reduced IL-12 secretion in Caco-2 cells and gene expression of IL-12, p35, IL-8, and tumor necrosis factor α (TNF-α) such as the inhibition of the translocation of NF-ĸB factor to the nucleus [60]. This direct effect was the peroxisome proliferator-activated receptor gamma (PPARγ) and peptidoglycan recognition protein 3 (PGlyRP3) dependent. To assess direct effects of prebiotics, Ortega-González et al. also tested prebiotics in IEC in vitro [61]. Prebiotics promoted the production of growth-related oncogene (GROα), monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 2 (MIP2) with an efficacy that was 50%–80% that of lipopolysaccharides (LPS). Interestingly, the response was markedly decreased by Toll like receptor 4 (TLR4) gene knockdown highlighting that prebiotics are TLR4 ligands in IEC. This was confirmed in a study where treatment of epithelial cells with FOS regulated the expression of TLR-modulated genes including IL-10, TNF-α, CXCL-8 and CXCL-1 [62].
Moreover, dietary supplementation with a synbiotic (prebiotics short chain (sc) GOS/ long chain (lc) FOS in combination with probiotic Bifidobacterium breve M-16V) increases galectin-9 on the surface of IEC and circulating galectin-9 levels in mice and humans. These observations were correlated with a reduction of acute allergic skin reaction and mast cell degranulation [63]. Galectin-9 was shown to decrease mast cell degranulation and promotes Th1 and Treg responses. This study highlighted that a diet enriched with a synbiotic leads to the prevention of allergic symptoms [63].
To demonstrate the direct effects of prebiotics on the maintenance of epithelial barrier function, a study by Wu et al. investigated the application of prebiotics onto immortalized gut-derived epithelial cell lines and human intestinal organoids in the absence of microbes and in the context of epithelial injury caused by a non-invasive human enteric bacterial pathogen [64]. Prebiotics directly promoted barrier integrity to prevent pathogen-induced barrier disruptions involving the induction of select tight junction proteins through a protein kinase C (PKC) δ-dependent mechanism. Réquilé et al. confirmed the effects of prebiotics on the tight junction gene expression [65]. These results demonstrate a specific and direct host-nutrient interaction and elucidate a novel mechanism whereby prebiotics maintain gut homeostasis to protect the host against challenge by enteric pathogens or allergens.
Another study has highlighted the interactions between epithelial cells and DC regulation by prebiotics [66]. The addition of prebiotic alone in the culture medium of DC did not modify their secretion. However, the addition of the epithelial cell culture supernatant incubated with prebiotics on DC increases the IL-10/IL-12 secretion ratio by DC. This suggests that prebiotics induce tolerogenic DCs mediated by molecules secreted by epithelial cells. The interaction of prebiotics with epithelial cells and DCs led to changes in the polarization of T cells. Interestingly, the different fibers tested had differential effects on T-cell polarization. GOS, chicory inulin, wheat arabinoxylan, and barley -glucan increased the production of the Th1 cytokine IFN-γ, whereas the Th1 cytokine TNF-α was decreased by GOS and wheat arabinoxylan. Barley-glucan increased the Th1 cytokine IL-2 and suppressed Th2 responses. GOS was the only prebiotic able to promote functional Treg (increase of IL10 secretion). They conclude that direct modulation of the IECs-DCs crosstalk can induce a regulatory immune phenotype and selecting dietary fibers could be essential for future clinical trials on efficacy in allergy management.
In summary, these studies demonstrate that prebiotics modulate host cell signaling to promote epithelial barrier integrity by direct effects on the intestinal mucosa.
Skin Epithelial Cells
It has been shown in mice, that GOS supplementation prevented trans epidermal water loss and UV-induced erythema via dermal expression of cell adhesion and matrix formation markers CD44, metallopeptidase inhibitor 1 (TIMP)-1, and collagen type 1(Col1), thereby improving the skin’s barrier properties [67]. It has also been shown in an AD mouse model that skin inflammatory cell infiltrations (such as skin Th2-related cytokines, TSLP and IL-4) were significantly reduced by treatments with FOS [68]. Moreover, CD4+ Foxp3+ Treg cells were significantly increased in skin lymph nodes. Finally, also in a mouse model of AD, supplementation with prebiotics (Konjac glucomannan) inhibits a scratching behavior and skin inflammatory immune responses by preventing germline class-switching and IgE production [69]. Prebiotic dietary supplementation significantly suppressed eczematous skin lesions, dermal mastocytosis and eosinophilia. Concomitantly, cutaneous overproductions of substance P, IL-10, IL-4, and TNF-α were all inhibited [70].
The prebiotic effects on skin epithelial cells in humans were confirmed in a double-blind, randomized trial on adult healthy subjects divided into two groups, control and GOS oral supplementation for 12 weeks [71]. The authors reported that GOS supplementation was beneficial to the skin characterized by better corneometer values and reduced transepidermal water loss (TEWL). In addition, the differences in total and percentage of wrinkle areas between the two groups were statistically significant after 12 weeks of GOS treatment. Finally, GOS can also prevent keratin depletion caused by phenolic compounds. In another trial involving patients with skin damage due to diabetes, 4 weeks of daily application of emollient containing prebiotics improved biophysical parameters of the epidermis [72]. They observed a decrease of the TEWL and skin dryness, and the restoration of skin sebum levels. Normalization of skin pH was suggested as the beneficial mechanism through improved epidermal skin integrity and limitation of bacterial infection.
In conclusion, both oral supplementation and cutaneous application with prebiotics have an impact on the biophysical parameters of the epidermis, improving the skin’s barrier properties. Knowing that the skin is an important route for allergen sensitization in AD infants [73], it would be interesting to study the effect of prebiotics application on the skin in high-atopy-risk infants to potentially reduce the risk of allergen skin sensitization.
Lung Epithelial Cells
In asthmatic airways, repeated epithelial damage and repair occur and the disrupted epithelial barrier and epithelial dysfunction are crucial in the induction and maintenance of asthma symptoms. No current therapy directly targets this process. Michael et al. have shown that treatment with prebiotics (Mannan Derived from Saccharomyces cerevisiae) stimulated cell spreading and facilitates wound repair in human bronchial epithelium, involving mannose receptors [74]. Prebiotics also increased the expression and activation of Krüppel-like factors (KLFs) 4 and 5, key transcription factors for epithelial cell differentiation, survival, and proliferation.
IS
The intestinal IS, also called gut-associated lymphoid tissue (GALT), is a secondary lymphoid organ involved in the processing of antigens that interact with the gut mucosa and the diffusion of the immune response. Prebiotics can be absorbed through the intestinal barrier and may thus be in direct contact with circulating IS cells. Inulin and FOS induce the secretion of IL-10, IL-1β and TNF-α by blood monocytes [75]. This secretion is due to the activation of the NF-κB pathway by the binding of the TLR4. In contrast, FOS and inulin do not have a significant impact on cytokine secretion by T lymphocytes. Possible direct effects of prebiotics are thought to entail ligation of pathogen recognition receptors (PRRs) on the surface of intestine DCs [76]. These PRRs involved in prebiotics signaling include TLRs, C-type lectin receptors (CLRs), NOD-like receptors (NLRs), and galectins. On DC derived from human blood monocytes, GOS and FOS induce IL-10 secretion stimulated by TLR4 binding [77]. Increased secretion of IL-10 by DC leads to the induction of regulatory Foxp3+ T cells. However, Perdijk et al. have shown that HMOs (6′-SL and 2′-FL) and GOS do not alter DC differentiation or maturation of in vitro differentiated DC types [78]. It was also shown that prebiotics supplementation in rats acted at the level of GALT enhancing the production of IL-10 and IFN-γ by CD4+ T lymphocytes in Peyer’s patches as well as the production of IgA in the caecum, compared with controls [79]. Caecal IgA secretion was shown to be dependent on the degree of polymerization of prebiotics [80]. Another study on mice also showed that FOS supplementation increased the secretion of IFN-γ and IL-10 in CD4+ T cells derived from Peyer’s patches [81].
