Your browser does not fully support modern features. Please upgrade for a smoother experience.
Subjects:
Virology
1. Introduction
The genus Enterovirus of the family Picornaviridae comprises many essential pathogens associated with human and mammalian diseases. This genus is classified into four groups: polioviruses, Coxsackie A viruses (CA), Coxsackie B viruses (CB), and echovirus. It contains fifteen species, encompassing four human enteroviruses (A-D), eight animal Enterovirus species (E-L), and three rhinoviruses (A-C). Enterovirus D68 (EV-D68) is a member of the Enterovirus D group of the Picornaviridae family. It was initially isolated and characterized from the throat swab samples taken from four Californian children with pneumonia and bronchiolitis in 1962 [1], giving rise to four distinct virus strains: Fermon, Franklin, Robison, and Rhyne. In recent years, there has been a gradual emergence of new representative strains, such as US/MO/14-18947(MO), US/KY/14-18953(KY), and others.
According to the statistics of the US National Enterovirus Surveillance System (NESS), only 26 sporadic cases of EV-D68 were reported from 1970 to 2005 [2]. Despite the fact that EV-D68-associated infection was previously considered infrequent, since 2005 it has undergone a worldwide burst of growth in the USA, Asia, and Europe, with an ever-increasing incidence over the past decade and a half [3]. In 2014, the United States experienced the largest upswing in EV-D68 infection: 1153 cases of EV-D68 were diagnosed and associated with severe respiratory symptoms [2]. A number of retrospective studies later also verified that a high prevalence of EV-D68 infection existed in Europe during the same period [4]. The most common clinical symptom of EV-D68 infection is respiratory illness [5]. As a non-polio enterovirus, EV-D68 was found to have a temporal association with a polio-like neurological disorder known as acute flaccid myelitis (AFM), with symptoms such as dysneuria and muscle weakness, and a causal link is being investigated by researchers [6]. This phenomenon suggests a potential for EV-D68 to induce nervous system disease [7], arousing widespread concern among health authorities and the public.
The genome of EV-D68 contains a positive-sense single-stranded RNA of about 7.6 kb. Like other picornaviruses, EV-D68 consists of a single open reading frame (ORF) flanked by untranslated regions (UTR) at each end [8], encoding a precursor polyprotein that is further cleaved autocatalytically to yield four structural proteins (VP1, VP2, VP3, and VP4) and seven non-structural proteins (2A, 2B, 2C, 3A, 3B, 3C, and 3D) (Figure 1) [8]. VP1, which contains the serotype-specific neutralization BC loop site, is used to distinguish among virus serotypes and to detect newly emerging strains [9]. Viral protein 2C has been reported to play an important role in the processes of infection and viral-host interaction, making it a promising target for anti-EV-D68 drugs. The selective serotonin reuptake inhibitor fluoxetine (Prozac) inhibits human enterovirus by targeting protein 2C and interfering with viral RNA replication [10]. However, fluoxetine has proven to be ineffective in improving the neurologic outcomes of EV-D68-associated AFM in a clinical trial [11]. Recently validated as a novel antiviral target, 2Apro is potently inhibited by telaprevir, an FDA-approved drug used for the treatment of hepatitis C virus infection [12]. The 3C protease contributes to the life cycle of viral infection and replication by inhibiting host innate immune responses; thus, it is also a potential target for antiviral drugs [13,14,15].
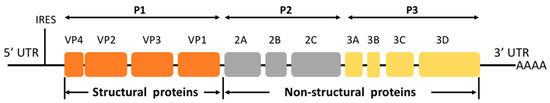
Figure 1. Schematic representation of the enterovirus D68 genome.
Recent studies on EV-D68 have mainly focused on epidemiology; the lack of basic research on its virologic characteristics and pathogenesis have hindered our further understanding of this virus. In this review, we will discuss advances regarding EV-D68’s genetic characteristics, infection mechanisms, interaction with hosts, and relationships with neurological diseases in order to provide useful information for future research into the pathogenesis and antiviral therapeutics of EV-D68.
2. Genetic Characteristics of EV-D68
EV-D68 is mainly divided into four subtypes and many branches, including the original classical subtypes and recent epidemic-related branches: Clade A, Clade B, Clade C, and Clade D (Figure 2). The location and corresponding year of specimen collection of EV-D68 VP1 sequences have revealed that EV-D68 subtype Clade A, Clade B, and Clade C are prevalent in multiple regions of American, Europe, and Asia. Several viral subtypes have often co-circulated, but Clade C is the least common of the subtypes. The major 2014 outbreak and currently prevalent strains in the United States are represented by Clade A1, Clade B1, Clade B4, and Clade B5 subtypes, whereas, also in 2014, European countries, such as Italy and the Netherlands, found confirmed cases of Clade A1, Clade A2(which has been reclassified to Clade D since 2016), Clade B1, and Clade B2 infection. In Canada, the major prevalent strain has been Clade B2. In Asian countries such as Thailand and Japan, the detection of Clade B and Clade C have been reported, whereas the main subtypes in China have been Clade A2 (which has been reclassified to Clade D since 2016) and Clade B2 [16]. The emergence of EV-D68 subclade D1 was recently reported in France and northern Italy, from August to November, 2018 [17,18]. Xiang et al. [18] reported that genogroup replacement has occurred in China since 2006. Before August 2011, Clade A was predominant in EV-D68-infected cases in China, and other strains were only scattered. Clade B emerged in October 2011 and co-circulated with Clade A until November 2013. However, in 2014, the circulating strains shifted from Clade A to B [19]. Genogroup replacement is one of the major causes of new outbreaks; fortunately, the genogroup replacement in China did not incur an EV-D68 upswing [19].
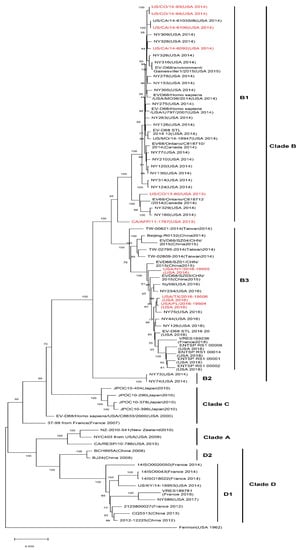
Figure 2. Phylogenetic tree of EV-D68 including acute flaccid myelitis (AFM)-related strains. A maximum likelihood phylogenetic tree of EV-D68 strains based on nearly complete polyprotein gene (1-6388 nt of coding sequences) was constructed using the bootstrap method with 1000 replications based on the Tamura–Nei model in MEGA X. The representative circulated EV-D68 strains during past years were collected in NCBI’s GenBank sequence database (n = 76). AFM-related EV-D68 strains are marked in red. AFM-associated stains are mostly in subclade B1 and B3. Branch lengths are drawn proportionally to the number of nucleotide substitutions per position.
Apart from the virus itself, community immunity, and especially the pre-existing virus serum neutralizing antibody (NAbs) in the community, are very likely to influence the spread and prevalence of the virus. Research on viral NAbs of specific populations can trace the history of viral infection and predict the susceptibility of a population to certain viruses. Xiang et al. [20] collected serum samples from individuals with EV-D68 infection in Beijing, China from 2004 to 2011, tested the seroprevalence of EV-D68 NAbs, and found that the level of EV-D68 NAbs in the Chinese community in 2004 was remarkably lower in all age groups than in 2009. Furthermore, from 2007 to 2011, the NAbs against EV-D68 in the population increased over time. Meanwhile, researchers also found that the NAbs actually can inhibit virus replication. Thus, the high level of EV-D68 NAbs in the Chinese community could be one of the reasons why EV-D68 infection in China appears only sporadically instead of in the form of outbreaks.
Recombination and mutations caused by the error-prone polymerase, human immune response, and population polymorphism have been recognized as the main mechanisms for enterovirus evolution [21]. It has been reported that the US EV-D68 outbreak in 2014 was caused by a novel clade [7,22]; researchers detected six kinds of gene mutation, M291T, V341A, T860N, D297N, S1108G, and R2005K in a distinct clade B1 of EV-D68 strains, which were associated with the neurovirulence of acute flaccid myelitis (AFM) in American strains [7,22]. Moreover, through comparative analyses of EV-D68 2014 outbreak isolate sequences, Zhang et al. also identified that AFM/AFP-associated isolates belong to a single phylogenetic subclade, B1, with 12 substitutions that carry the same residues observed at equivalent positions in paralysis-causing enterovirus such as poliovirus, EV-D71, and EV-A71 [23]. Moreover, subclade B3, now the most prevalent EV-D68, is also reported to be associated with AFM [24]. Later, Huang et al. discovered three variants (C187T, C3277A, and A4020G) that differ from those previously identified through comparative genomic analysis. C3277A and A4020G cause amino acid substitutions T860N and S1108G, respectively, which are cleavage sites for the viral proteases 2A and 3C. The mutation of these sites alters the cleavage efficiency, leading to an increased rate of viral transmission and replication [5]. However, there have been cases of infection by EV-D68 strains with neurovirulence-related mutations that have only shown non-neurologic symptoms such as respiratory illness [7]. This result suggests that viral gene polymorphism is necessary to cause nervous system symptoms such as AFM, but other factors also need to be considered. Nevertheless, only one case was reported in China in 2011 in which the individual was infected by the mutant strain that had the six substitutions mentioned above. In 2014, a unique R220A mutation was detected in the EV-D68 strain prevalent in China [18], which might also result in changes in virulence. As in other enteroviruses, the loop sequences of the X and Y domains in the 3’-UTR of EV-D68 are complementary, but Xiang et al. found that the X loop sequences of the US 2014 outbreak EV-D68 strains were not complementary but instead identical to loop sequences of the Y domain, indicating that the structure of the 3’-UTR of the viral genome can affect viral replication. Meanwhile, all the epidemic EV-D68 American strains have C-U, C-A mutations in the 3’-UTR, a finding that may influence the phenotypes of the virus [18].
Existing epidemiological evidence indicates that the US has the highest prevalence of EV-D68. However, in recent years, cases of EV-D68 infection in Europe, Asia, and other countries have continued to increase. The evolution of EV-D68 exhibits geographic and temporal diversity, and changes in genotype largely affect the pathogenicity and epidemic characteristics of the virus.
Conclusions
In recent years, infections with the once rarely reported enterovirus D68 have increased remarkably worldwide, accompanied by severe respiratory illness and neurological complications. Infection cases are mainly diagnosed in children and young adults and have raised widespread concern. Discovery of the cellular receptors, sialic acid, and neuron-specific receptor ICAM-5, has proven essential for uncovering the mechanisms of EV-D68 pathogenesis. Meanwhile, studies of the effects of non-structural proteins on virus–host interactions have revealed that the virus can promote replication by altering the cell cycle and escaping innate immunity. Thus, it appears that the development of targeted therapeutic interventions to treat EV-D68 infection that involve inhibitors of the non-structural proteins 2C, 3C, and 2A will prove worthwhile. In addition, investigation of the association between EV-D68 and AFM is important for further elucidating the clinical features of EV-D68 and revealing its virologic characteristics. However, much is still unknown about EV-D68: Its nosogenesis, the role of many viral proteins in the replication cycle of the virus, a more comprehensive, detailed picture of the interplay between EV-D68 and host immunity, genetic variation in EV-D68, and the potential for antiviral therapeutics all await further investigation.
