Fermented foods identify cultures and civilizations. History, climate and the particulars of local production of raw materials have urged humanity to exploit various pathways of fermentation to produce a wide variety of traditional edible products which represent adaptations to specific conditions.
- fermented foods
- microbiology
- health
- oral health
- LAB
- bacteriocins
- antibiotic resistance
- fermented diet
- caries control
- tooth erosion control
1. Introduction
Louis Pasteur discussed the issue of fermentation [1][2]. He obviously suspected—and, in some cases, he knew—that fermentative processes are diverse and complicated. Indeed, although modern science recognizes more than one type of fermentation, a general definition should define fermentation as a biochemical process through which most microorganisms decompose carbohydrates to produce energy under anaerobic conditions [3][4][5]. According to Pasteur, “Fermentation is life in the absence of oxygen” [1]. In this somewhat obscure and phenomenological term, various types of fermentation are included, such as yeast fermentation, lactic acid fermentation, butyric acid fermentation, propionic acid fermentation and acetic acid fermentation. The diversity of the phenomenon leads to a variety of end products, which include CO2, ethanol, organic acids and other organic molecules. It is many of these products that make fermentation so useful and interesting both to science and industry [3][4][5][6][7].
The process of carbohydrate decomposition creates a flow of protons and electrons. In aerobic conditions, the final receptor is oxygen (respiration), andin anaerobic conditions, the final receptors are other organic molecules, such as pyruvate or acetyl CoA (fermentation). Fermentation yields much less energy than respiration. The progressive oxidation of sugars involves the transfer of hydrogen ions from intermediate products in the pathway to the final receptor. The Embden–Meyerhof–Parnas pathway (EMP) is utilized in most cases to decompose glucose to pyruvate (homofermentive strains). The Entner–Doudoroff pathway decomposes lactose molecules, while the heterofermentive strains decompose pentoses and other sugars through the phosphoacetylase pathway. The end products depend on the substrate as well as the microorganism species and strain (Figure 1 and Table 1). Different species and different genera have different capacities of fermentation [3][6][7]. For example, E. coli has limited fermentation capacities regarding the variety of end products as compared to lactic acid bacteria (LAB). On the other hand, beneficial fermentation properties can be engineered. By inserting a lactic acid dehydrogenase gene from a bovine muscle, Saccharomyces cerevisiae can produce lactic acid in levels similar tothose of LAB [8].
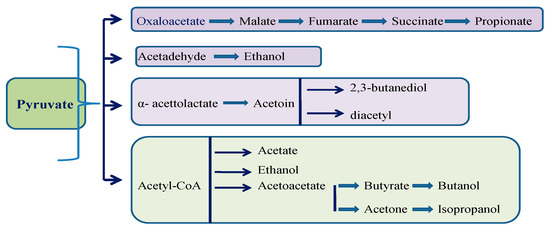
Figure 1. The diversity of fermentations. One molecule of glucose through glycolysis results in two molecules of pyruvate. Under anaerobic conditions and depending on the microorganism involved, fermentation can continue in certain pathways.
Table 1. Most common fermentations and the implicated microorganisms.
| Type of Fermentation | Microorganisms Involved | Food/Environment | Major End Products |
|---|---|---|---|
| Alcohol fermentation | Yeasts | Wine, beer, sourdough | 2 glucose → 2 glycerol + acetic acid + ethanol |
| Lactic acid (homofermentation) | Lactic acid bacteria (Lactobacillus spp. etc.) | Dairy products, fermented meats and fermented vegetables etc. | Glucose → 2 lactic acid + 1ATP (Embden-Meyerhof-Parnas) |
| Lactic acid (heterofermentation) | Lactic acid bacteria (Lactobacillus spp. etc.) | 5 and 6 carbon sugars → lactic acid + acetic acid+ ethanol +1ATP | |
| Butyric acid | Clostridium spp., Butyrivibrio spp., Bacillus spp. and other anaerobes | Marsh sediments, sewage systems | 4 glucose → 2 acetate + 3 butyrate → butyric acid, butanol, acetoine, isopropanol, acetate, ethanol, 2.3-butadienol |
| Mixed acid | Enterobacteriaceae (Escherichia spp., Enterobacter spp., Salmonella spp., Klebsiella spp., Shigella spp. etc.) | Human and animal digestive tract, fresh water | Glucose → acetic acid, formic acid, lactic acid, succinic acid, ethanol |
| Propionic acid | Propionebacterium spp., Veilonella spp., Bacteroides spp., some Clostridia spp. | Dairy products | Glucose, glycerol, lactate → propionate, acetate, |
| Acetic acid | Acetobacter spp., Gluconobacter spp., Bacillus subtilis |
Acetic acid industry | Oxidation of sugars, sugar alcohols, ethanol →acetic acid |
Microorganisms and carbohydrates are abundant in nature and fermentation occurs in every anaerobic environment. It follows that it is not a question of “if’ but a question of “when” and “how” early humans discovered and harvested the benefits of fermentations in the domain of food and beverage. Near Lake Neuchatel in Switzerland, archaeologists discovered the remains of cheese manufacturing from 6000 BCE [9]. Homer refers to Cyclops Polyphemus as a cheese producer, while in many cultures, such as the Mongols, the Tuareg, the Bedouin, the Tibetans many central Africa tribes and Amazon natives, among others, strong evidence exists of fermented curds production in the antiquity [10][11]. Bread is depicted in ancient Egypt scrolls aging from 2700–2500 BCE. In the third century CE, 72 kinds of bread were listed in Athens [11]. Finally, wine must have been “invented” somewhere between the Black Sea and the Caucasus mountains from 6000 to 3500 BCE. Apparently, someone accidentally left crashed grapes in a container and tasted them much later [12][13][14]. A similar theory is presented for beer brewery, but in this case, meshed cereals with water were left the container [15]. In ancient Egypt, the kneading and baking facilities were beside the beer breweries, obviously both using Saccharomyces cerevisiae [15][16].
2. Safety Issues
One could argue that microbiomes can protect their food ecosystems themselves. Indeed, it seems that microbiomes can deploy certain defence lines and hence rapidly transform their environment in such a way that it finally becomes hostile to most other microorganisms. The key words are “finally” and “most.” It is true that fermentation itself serves as the first line of defence by rapidly decreasing the pH values (the involved microorganisms are also called “fast acidifiers”) through the production of organic acids (e.g., lactic acid) and also by eliminating the carbohydrates of the environment, thus depriving the competitive bacteria of nutrients. However, this process—no matter how fast it proceeds—takes some time, and this provides a window of opportunity to various spoilage intruders or pathogens. This is particularly true for fresh cheeses of artisan origin. Various Brucella strains manage to survive in such products, causing serious foodborne illnesses, although they are eliminated in cheeses with a ripening period longer than 2 months [17]. Normally, by the end of the ripening process, most pathogens and spoilage bacteria will be destroyed or permanently inactivated due to the low pH, deprivation of nutrients and antibacterial activity of certain substances produced by the fermentation flora. Staphylococcal species, on the other hand, survive throughout the fermentation process, though a healthy microbiome can control effectively their populations [18].
2.1. Bacteriocins
Besides organic acids, certain species of microbiome communities, and particularly the lactic acid bacteria (LAB), excrete molecules with antimicrobial action such as hydrogen peroxide, acetaldehyde and bacteriocins. These molecules—products of the bacterial cell metabolism—have been proven effective against pathogens and spoilage bacteria both in vivo and in vitro. The production of bacteriocins is among the criteria for a strain to be characterized as probiotic [19].
Bacteriocins are proteinaceous molecules (peptides) able of destroying other bacteria. They are synthesized in the ribosomes and are excreted extracellularly, killing mainly—but not exclusively—Gram-positive bacteria [20][21][22]. They have a narrow or broad spectrum of activity depending on the number of sensitive species. All bacteriocins do not possess the same antibacterial capacity, and all bacteriocin-producing bacteria do not produce the same amount of bacteriocins of the same potency. Their activity depends on their exact chemical structure as well as the susceptibility of the target cell [23]. These substances attract increasing attention in the food industry because they are a natural means of preservation of products against pathogens and spoilage bacteria. The utilization of bacteriocins can potentially lead to milder treatments or minimal processing. Bacteriocins are not toxic and they do not alter the sensorial characteristics of the products. These advantages meet the expectations of the consumers for “natural” foods and less chemicals. They also satisfy the necessity of the food industry to satisfy these demands. Thus, bacteriocins fit perfectly into the concept of “biopreservation” [24].
Bacteriocins can either be added directly as chemical substances to the products or can be added indirectly through LAB cultures, although it seems that the latter case is not an optimal strategy because the additional LAB could ferment the carbohydrates of the product, thus altering its properties. Nisin, pediosin, enterocins AS-48 and 416K1, bificin C6165 and bovicin are bacteriocins which have been studied for their antimicrobial action. So far, only pediosin and nisin have received official approvals as food additives, yet the research in that scientific field is ongoing and the future of bacteriocins in the food industry is promising [22].
2.2. Antibiotic Resistance
Resistance to antibiotics (AR) is globally recognized as an emerging serious threat to human health and as a food safety problem (FAO/WHO 2018, WHO 2020) [25]. The term refers to bacterial strains (pathogens or not) that are resistant to one or more antibiotics (multidrug resistant, MDR). It is estimated that AR causes at least 700,000 deaths worldwide [26]. In the EU, there is an officially expressed concern for the observed increased resistance to first-line antibiotics such as carbapenems and quinolones [27]. In the 2019 CDC report on AR, it was estimated that, in the US, 2.8 million people are infected by resistant bacteria while 35,000 die as a result of these infections (CDC 2020) [28].
Foods, and particularly fermented foods, are ecosystems favouring bacterial growth. The ecology of food natural flora is very complicated and can originate from various niches. Fermentations’ microbiomes are a heterogenous group in the sense that the bacterial species colonizing such foods occur from diverse origins, such as plants and their soil, the animals’ udder or gut, the environment in which the animals live (terrestrial or aquatic), the facilities of the food processing (dairies, abattoirs, etc.) and the retail and distribution networks. In this way, various ecological niches are represented in the biota of fermented milks, meats and vegetables. In these niches, bacteria and AR genes co-circulate. The so-called mobile genetic elements (MGE), R plasmids, integrons and transposons, as well as bacteriophages, carry the genetic determinants of AR and disperse them among bacterial communities [29]. Hence, AR is acquired from pathogenic and non pathogenic bacteria. In the former case, the threat to the consumer’s health is straightforward and represents a medical emergency. However, it is the latter case that poses a more subtle and potentially more dangerous health hazard. Non pathogens and commensal bacteria such as these that are found in the microbiomes can easily acquire resistance genes from other bacteria through MGE and can confer them to the consumer’s intestinal microbiome. Thus, even a non-resistant pathogen (foodborne or not) can receive resistance genes in the human digestive tract [29].
LAB bacterial species dominate the flora of fermented foods and most of them, such as Lactobacillus spp., have been shown to be resistant to a variety of antimicrobial substances representing all categories of antibiotics, thus posing a potential danger [30]. Regulation through legislation for the prudent use of antibiotics in agriculture, husbandry and aquacultures is necessary to tackle this issue. Regulation should also be imposed in the food industry concerning the resistance to antibiotics.
Antibiotics are used as therapeutic means against infectious disease in humans, animals and aquaculture. However, the haphazard and extensive use of antibiotics may lead toantibiotic-resistant bacteria [31]. The development of resistance to antibiotics can be established by several mechanisms, that is, by enzymatic degradation, by modifying the target of the antibiotic, by affecting the permeability of the bacterial cell wall and by creating alternative pathways to elude the activity of the antibiotic [32]. Some species exhibit inherited or innate resistance to certain antibiotics, but such resistance cannot be transferred to other bacteria. Acquired antibiotic resistance is observed when bacteria acquire genes encoding a resistance mechanism either from other bacteria or by mutations [33].
Fermented foods and beverages are produced worldwide, and they are valued for their sensorial and nutritional characteristics. Numerous studies have shown that the beneficial bacteria of these ferments are also carriers of resistance to antibiotics [34]. The food chain is an immense route and the fermented foods a subroute through which the determinants of resistance to antibiotics are conferred to pathogens and to commensal bacteria [35].
LAB particularly is abundant in fermented foods and may act as reservoirs of antibiotic resistance genes, which, in turn, can be horizontally transmitted to pathogens and to the consumers’ microbiome via the food chain [36][37].
Antimicrobial resistance genes can be transferred into microbial communities by mobile genetic elements (MGE) such as plasmids, transposons, integrons, cassettes or bacteriophages [38]. The European Scientific Committee on Animal Nutrition and the European Food Safety Authority recommend that LAB strains consumed daily should lack acquired or transferable antimicrobial resistance genes in order to be considered safe for human and animal consumption [39].
3. Conclusions
Fermentations dominate the history and present of nutrition in the sense that, in one form or another, most humans eat (or drink) fermented foods daily. There is a vast variety of fermented foods and drinks throughout the world, some of which were indicatively discussed. The multitude of microorganisms involved in fermentations—associated with complicated metabolic pathways—is more than impressive. Apart from their nutritional effects, fermented foods enhance and protect the health of the consumer in more than one way. Most of these microorganisms extend their action into digestive tract of the consumer, conferring serious benefits to his/her health. This probiotic effect has been extensively verified in vivo and in vitro by numerous reports. Regular consumption of fermented foods reduces the risk of certain types of cancer, protects the health of the gastrointestinal tract and the urogenital tract, alleviates symptoms of diseases such as Crohn’s disease and irritable bowel disease and enhances the immune system. There are strong indications of a positive impact to certain psychological and behavioural disorders. There is also a certain beneficial effect of their daily use to the oral health. By lowering the pH value in the oral cavity, they prevent dental caries and the erosion of teeth while contributing to the treatment of periodontitis and halitosis. The lowering of the pH value of the fermented products is a defence mechanism of the microbiome, since most antagonistic bacteria cannot survive at low pH values. Bacteriocins are an effective means of overcoming other bacteria. These substances are a very promising tool in the food industry because they are a natural way of preservation, fully compatible with consumers’ expectations for a mild treatment of food. Yet, the increasing incidence of resistant strains in the microbiomes is a trend that should be addressed by the scientific community.
References
- Modern History Sourcebook: Louis Pasteur (1822–1895): From the Physiological Theory of Fermentation by Louis Pasteur. 1879. Available online: https://sourcebooks.fordham.edu/mod/1879pasteur-ferment.asp (accessed on 21 January 2020).
- Barnett, J. Beginnings of microbiology and biochemistry: The contribution of yeast research. Microbiology 2003, 149, 557–567.
- Albert, G.; Moat, J.W.; Foster, M.P.S. Microbial Physiology, 4th ed.; Wiley-Liss Inc.: New York, NY, USA, 2002; pp. 412–431.
- Thomas, J.; Montville, K.R.M. Food Microbiology. An Introduction, 2nd ed.; ASM Press: Washington, DC, USA, 2008; pp. 244–245.
- Toussaint-Samat, M. A History of Food; Wiley-Blackwell: Oxford, UK, 2009; pp. 103, 201, 223.
- Angold, R.; Beech, G.; Taggart, J. Food Biotechnology: Cambridge Studies in Biotechnology 7; Cambridge University Press: Cambridge, UK, 1989.
- Caballero, B.; Trugo, L.C.; Finglas, P.M. (Eds.) Encyclopaedia of Food Sciences and Nutrition; Academic Press: Oxford, UK, 2003.
- Tokuhiro, K.; Ishida, N.; Nagamori, E.; Saitoh, S.; Onishi, T.; Kondo, A.; Takahashi, H. Double mutation of the PDC1 and ADH1 genes improves lactate production in the yeast Saccharomyces cerevisiae expressing the bovine lactate dehydrogenase gene. Appl. Microbiol. Biotechnol. 2009, 82, 883–890.
- Frutiger, M. The History of Cheese in Switzerland. 2017. Available online: https://www.swissclubnsw.com/single-post/2017/04/26/The-History-of-Cheese-in-Switzerland (accessed on 26 April 2017).
- Homer, ’The Odyssey’ Bk V:192-261. Available online: https://www.poetryintranslation.com/PITBR/Greek/Odhome.php (accessed on 8 December 2004).
- Prajapati, J.B.; Nair, B.M. The history of fermented foods. In Fermented Functional Foods; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA; New York, NY, USA; London, UK; Washington, DC, USA, 2003; pp. 1–25.
- McGovern, P.E. Ancient Wine: The Search for the Origins of Viniculture; Princeton Univ Press: Princeton, NJ, USA, 2007.
- McGovern, P.E. Uncorking the Past: The Quest for Wine, Beer, and Other Alcoholic Beverages; Univ. of California Press: Berkeley, CA, USA, 2010.
- McGovern, P.E.; Mirzoian, A.; Hall, G.R. Ancient Egyptian herbal wines. Proc. Natl. Acad. Sci. USA 2009, 106, 7361–7366.
- Hukins, W.R. Microbiology and Technology of Fermented Foods; John Wiley & Sons: Hoboken, NJ, USA, 2006.
- Hornsey, S.I. A History of Beer and Brewing; Royal Society of Chemistry: London, UK, 2003.
- Falenski, A.; Mayer-Scholl, A.; Filter, M.; Göllner, C.; Appel, B.; Nöckler, K. Survival of Brucella spp. in mineral water, milk and yogurt. Int. J. Food Microbiol. 2011, 145, 326–330.
- Pazakova, J.; Turek, P.; Laciakova, A. The survival of Staphylococcus aureus during the fermentation and storage of yoghurt. J. Appl. Microbiol. 1997, 82, 659–662.
- Dobson, A.; Cotter, D.P.; Ross, R.P.; Hill, G. Bacteriocin production: A probiotic trait? Appl. Environ. Microbiol. 2012, 78, 1–6.
- Yang, E.; Fan, L.; Jiang, Y.; Doucette, C.; Fillmore, S. Antimicrobial activity of bacteriocin-producing lactic acid bacteria isolated from cheeses and yogurts. AMB Express 2012, 2, 48.
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–778.
- Barbosa, A.A.T.; Mantovani, H.C.; Jain, S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit. Rev. Biotechnol. 2017, 37, 852–864.
- Voidarou, C.; Alexopoulos, A.; Tsinas, A.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. Effectiveness of bacteriocin-producing Lactic acid bacteria and Bifidobacterium isolated from honeycombs against spoilage microorganisms and pathogens isolated from fruits and vegetables. Appl. Sci. 2020, 10, 7309.
- Borges, S.; Teixeira, P. Application of bacteriocins in food and health care. In Bacteriocins: Production, Applications and Safety; Nova Science Publishers: New York, NY, USA, 2016.
- Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 31 July 2020).
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44.
- Available online: https://www.efsa.europa.eu/en/news/antimicrobial-resistance-eu-infections-foodborne-bacteria-becoming-harder-treat (accessed on 3 March 2020).
- Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 31 December 2020).
- Sørum, H.; L’Abee-Lund, M.T. Antibiotic resistance in food-related bacteria--a result of interfering with the global web of bacterial genetics. Int. J. Food Microbiol. 2002, 78, 43–56.
- Rozos, G.; Voidarou, C.; Stavropoulou, E.; Skoufos, I.; Tzora, A.; Alexopoulos, A.; Bezirtzoglou, E. Biodiversity and microbial resistance of lactobacilli isolated from the traditional Greek cheese kopanisti. Front. Microbiol. 2018.
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433.
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; De Schaetzen, M.A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669.
- MacGowan, A.; Macnaughton, E. Antibiotic resistance. Medicine 2017, 45, 622–628.
- Anal, A.K.; Perpetuini, G.; Petchkongkaew, A.; Tan, R.; Avallone, S.; Tofalo, R.; Van Nguyen, H.; Chu-Ky, S.; Ho, P.H.; Phan, T.T.; et al. Food safety risks in traditional fermented food from South-East Asia. Food Control 2020, 109, 106922.
- Abriouel, H.; Knapp, C.W.; Gálvez, A.; Benomar, N. Chapter 29—Antibiotic resistance profile of microbes from traditional fermented foods. In Fermented Foods in Health and Disease Prevention; Frías, J., Martínez-Villaluenga, C., Peñas, E., Eds.; Academic Press: London, UK, 2017; pp. 675–704.
- Maidana, D.S.; Ficoseco, S.A.; Bassi, D.; Cocconcelli, P.S. Biodiversity and technological-functional potential of lactic acid bacteria isolated from spontaneously fermented chia sourdough. Int. J. Food Microbiol. 2020, 316, 108425.
- Zarzecka, U.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Starter cultures as a reservoir of antibiotic resistant microorganisms. LWT 2020, 127, 109424.
- Toscano, M.; Peroni, D.; De Vecchi, E.; Mattina, R.; Drago, L. Microbiological assessment of some powdered infant formulas: From quality to antibiotic resistance evaluation. J. Food Process. Technol. 2013, 4, 12.
- Available online: https://www.efsa.europa.eu/sites/default/files/consultation/120323.pdf (accessed on 31 December 2020).

