Your browser does not fully support modern features. Please upgrade for a smoother experience.
Subjects:
Allergy
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) belong to a group of gastrointestinal hormones called incretins. Insulin released from the beta cells of the islets of Langerhans after ingestion of food is the major regulator of GIP and GLP-1 secretion.
- glucose-dependent insulinotropic polypeptide
- glucagon-like peptide-1
- dipeptidyl peptidase-4
1. Physiology of GIP and GLP-1
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) belong to a group of gastrointestinal hormones called incretins. Insulin released from the beta cells of the islets of Langerhans after ingestion of food is the major regulator of GIP and GLP-1 secretion [1]. Both hormones show complementary action of the β-cells of the pancreas by acting through different but related receptors [2]. GLP-1 is secreted from endocrine L-cells mainly found in the distal ileum and colon, while GIP is released from K-cells located in the duodenum and jejunum [2]. GLP-1 secretion is stimulated by activation of a number of intracellular signals, including PKA (protein kinase A), PKC (protein kinase C), calcium, and MAPK (mitogen-activated protein kinase) [2]. GLP-1 release is regulated by cell membrane channels: glucose stimulates GLP-1 secretion via KATP (adenosine triphosphate-sensitive potassium) channel closure [3], while nonmetabolizable carbohydrates using mechanisms dependent on sodium-glucose cotransporters 1 and 3 [4]. Many forms of GLP-1 are released in vivo, including GLP-1(1-37) and GLP-1(1–36)NH2, which are inactive, and GLP-1(7–37) and GLP-1(7–36)NH2, which are biologically active. GLP-1(7–36)NH2 consists of the majority of GLP-1 circulating in the human body [5][6].
The human GIP gene comprises six exons and has been localized on the long arm of chromosome 17 [2]. Previous studies demonstrated that bioactive form of GIP (42-amino acid) is released from its 153-amino acid proGIP precursor via PC 1/3 (prohormone convertase 1/3)-dependent posttranslational cut [7]. The GIP sequence is highly conserved, with more than 90% amino-acid sequence identity in humans and other vertebrates. [2] The GIP receptor (GIPR) initially was isolated from a rat brain, but nowadays it is known that it is also expressed throughout the digestive tract, pituitary, lungs, trachea, kidneys, bones, thymus, spleen, endothelial cells, thyroid, adrenal cortex, testis, and central nervous system [8]. It was previously demonstrated that the secretion of GIP may increase via β-adrenergic stimulation, K+-mediated depolarization, rise of intracellular Ca2+ concentration, and activation of adenyl cyclase [9]. Studies have shown that ahead of gastrin, secretin, and cholecystokinin, GIP and GLP-1 are predominant incretin hormones in humans [1].
For the first time, the intestinal peptide GIP was isolated from porcine upper small intestine about 50 years ago [10], and it was discovered about 10 years later that two GLP-1 fragments were potent regulators of insulin secretion [11]. Both hormones are elevated after an oral glucose load or meal consumption in healthy humans and nowadays are commonly known for regulating glycemic level by increasing insulin secretion, inhibiting glucagon release, and delaying stomach emptying [12]. However, the increase in incretins after meals is disproportionately low in people with type 2 diabetes (DM 2) [13]. Although the reasons for this phenomenon are not fully explained, several mechanisms are postulated. Because insulin increase appears to be the main regulator of GIP and GLP-1 release, and insulin secretion in DM 2 patients is reduced due to beta cells dysfunction, it can be assumed that diminished effect of incretins in DM 2 can be associated with β-cell dysfunction rather than with a direct impairment of incretins secretion or activity [14][15]. In addition, acute elevation of glucose concentration (due to continuous insulin deficiency) in diabetic patients has been shown to reduce postprandial GLP-1 response [15][16]. Another postulated mechanism is specific loss of GIP activity. It may seem that glucose-induced insulin release impairment appears to be comparable to the corresponding GIP-induced insulin secretion defect. It is possible that the inability of GIP to increase insulin secretion during hyperglycemia is primarily due to the lack of glucose amplification of insulin release in DM 2 patients [17][18]. The insulinotropic GIP and GLP-1 inefficiency in diabetics is perhaps related with an additional (not yet explored) mechanism of action rather than with a specific defect in GIP regulation. Further research is required to clarify these mechanisms.
2. GIP and GLP-1—Importance in Medicine
For many years it was known that intravenous GIP or GLP-1 administration could normalize hyperglycemia in DM 2 patients [13]. Hence, GLP-1 receptor agonists (GLP-1 RAs) have been widely used as an oral treatment of DM 2 for several years [19][20]. Exenatide was introduced for the treatment of DM 2 in 2005 and liraglutide was approved for use four years later. [21] Besides these two, many other GLP-1 agonists like Semaglutide, Efpeglenatide, Dulaglutide, Albiglutide, and Lixisenatide have been recently synthesized [22]. Besides efficacy in reducing hyperglycemia, GLP-1 RAs have many additional benefits. One of their advantages over older insulin secretagogues, such as sulfonylureas or meglitinides, is lower risk of hypoglycemia development [23]. In addition, constant intake of GLP-1 stimulates weight loss and reduces appetite by inhibiting gastric emptying [23].
Apart from the undoubted impact of GIP and GLP-1 on carbohydrates metabolism, their receptors were also expressed in organs and cells such as duodenum, liver, kidneys, peripheral and central nervous system, adipocytes, osteoblasts, and myocytes [2][24].
2.1. Liver
2.2. Kidneys
The GLP-1 receptor (GLP-1R) is found expressed in kidneys and intravenous administration of GLP-1 in rats has both a diuretic and a natriuretic effect with increases in glomerular filtration rate and inhibition of sodium reabsorption in the proximal tubule. Moreover, by enhancing the excretion of water and salt, GLP-1 demonstrates antihypertensive action [27]. Additionally, intravenous infusion of GLP-1 reduces H+ (hydrogen) secretion and glomerular hyperfiltration and thus protects the kidneys [28].
2.3. Nervous System
In the central nervous system, GLP-1 inhibits the intake of food and fluids [29], decreases energy intake, and leads to weight loss by constant activation of satiety region in the hypothalamus [30]. Moreover, the anorectic effects of GLP-1 RAs have been described [31]. GLP-1R was also proved to be involved in many pathways for learning and memory abilities [32]. GLP-1 RAs have neuroprotective and anti-apoptotic effects on neuronal cells in a rodent model [33], and for this reason, GLP-1R can be proposed as a therapeutic target in many neurological disorders and neurodegenerative diseases. In the central nervous system, GIP is expressed mainly in the hippocampus; it has been suggested that administration of GIP induces proliferation of hippocampal progenitor cells and thus may enhance memory abilities and behavioral changes [34].
2.4. Adipose Tissue
Incretins may display both lipogenic and lipolytic effects in human adipocytes. The anabolic effects of GIP include promoting fatty acid synthesis, insulin-dependent incorporation of fatty acids into triglycerides, enhancement of lipoprotein lipase production, and reduction of glucagon-stimulated lipolysis [35][36]. However, GIP may also act lipolitically by improving glucose tolerance, enhancing insulin sensitivity and reducing obesity-related pancreatic β-cells hyperplasia [37].
2.5. Bones and Muscles
For the skeletal system, GIP is involved in bone formation and increases bone mineral density in rats [38]. These effects are mostly caused by increasing intracellular calcium (Ca2+) concentration. Additionally, GIP administration was found to be an inhibitor of bone resorption by retardation of osteoclasts proliferation [39]. In rat and human muscles, GLP-1 increases glycogen synthase activity and interferes with glucose metabolism [40].
2.6. Endocrine System
Finally, incretins are also involved in the secretory activity of endocrine glands. GLP-1 stimulates a release of thyroid-stimulating hormone (TSH), luteinizing hormone (LH), corticosterone and vasopressin, and modulates neuroendocrine cells in hypothalamic rats [41]. Although it has not been definitely confirmed that GIP regulates cortisol release in healthy subjects, abnormal expression of GIPR was found to be related with development of Cushing’s syndrome [42].
2.7. Inflammation
Moreover, studies describing the beneficial anti-inflammatory effect of GIP and GLP-1 can be found in the literature [43]. GIPR and GLP-1R are widespread expressed on many immune cells, and it was proved that GIP and GLP-1 can activate the immune system to restrain disease processes [44]. On the other hand, GLP-1 concentrations were found to be higher in critically ill patients with sepsis and positively correlated with proinflammatory markers [45].
3. Dipeptidyl Peptidase-4 (DPP-4) Role
Both GIP and GLP-1 are rapidly inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4) with a blood half-life of only a few minutes [13]. DPP-4, also known as CD 26 (cluster of differentiation 26), was discovered over 50 years ago [46]. DPP-4 belongs to the adipokines family and appears an important molecular biomarker that is strongly correlated with metabolic syndrome [47]. DPP-4 expression was proved to positively correlate with body mass index (BMI) and adipocyte size and activity in both subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) [47]. In the same study, circulating DPP-4 concentration in insulin-sensitive obese patients was significantly lower than in those with insulin resistance [47]. However, it has not yet been fully explained which tissue is the source of circulating DPP-4. It is also known that DPP-4 is involved in metabolism and release of broad range of molecules such as chemokines, vasoactive peptides, neurokinins, and growth factors [48]. DPP-4 is also associated with cancer biology and is useful as a marker of various tumors, with its level strongly correlated with neoplasia [49]. Additionally, DPP-4 inhibitors were found to have an anti-inflammatory effect via inhibition of monocyte activation and chemotaxis [50] and are involved into inhibition of the development of endothelial dysfunction and thus prevent atherogenesis in nondiabetic apolipoprotein E-deficient (ApoE−/−) mice [51].
Apart from pleiotropic actions, DPP-4 plays the most important role in glucose metabolism [52]. By breaking down endogenous incretins GIP and GLP-1, it increases plasma glucose level both in fasting and fed conditions and is also responsible for less postprandial insulin burst [53]. Therefore, drugs that are DPP-4 inhibitors delay the breakdown of endogenous incretins GIP and GLP-1 and are successfully used to combat insulin-resistance and DM 2 management [20].
GIP, GLP-1 and DPP-4 inhibitors have not only positive pleiotropic effects on the whole human body. Incretin-based therapy has recently been shown to be associated with an increased risk of pancreatic cancer in diabetes patients [54].
The currently known physiological effects of GIP, GLP-1, and DPP-4 on animal and human tissues are presented on Figure 1.
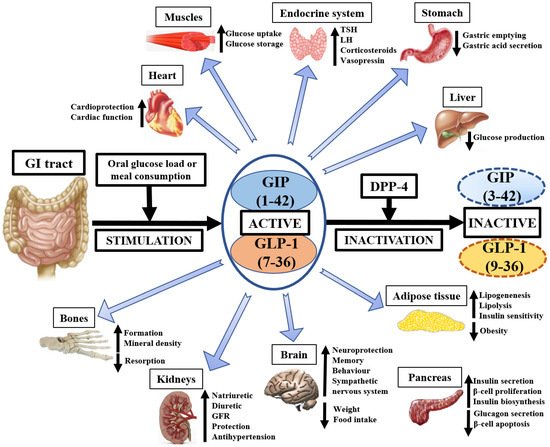
Figure 1. The pleiotropic physiological importance of GIP, GLP-1, and DPP-4 in medicine. GI—gastrointestinal, GIP—glucose-dependent insulinotropic polypeptide, GLP-1—glucagon-like peptide-1, DPP-4—dipeptidyl peptidase-4, GFR—glomerular filtration rate, TSH—thyroid-stimulating hormone, LH—luteinizing hormone.
References
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536.
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157.
- Reimann, F.; Gribble, F.M. Glucose-Sensing in Glucagon-Like Peptide-1-Secreting Cells. Diabetes 2002, 51, 2757–2763.
- Gribble, F.M.; Williams, L.; Simpson, A.K.; Reimann, F. A Novel Glucose-Sensing Mechanism Contributing to Glucagon-Like Peptide-1 Secretion From the GLUTag Cell Line. Diabetes 2003, 52, 1147–1154.
- Dhanvantari, S.; Seidah, N.G.; Brubaker, P.L. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol. Endocrinol. 1996, 10, 342–355.
- Ørskov, C.; Wettergren, A.; Holst, J.J. Biological Effects and Metabolic Rates of Glucagonlike Peptide-1 7-36 Amide and Glucagonlike Peptide-1 7-37 in Healthy Subjects Are Indistinguishable. Diabetes 1993, 42, 658–661.
- Ugleholdt, R.; Poulsen, M.L.; Holst, P.J.; Irminger, J.C.; Orskov, C.; Pedersen, J.; Rosenkilde, M.M.; Zhu, X.; Steiner, D.F.; Holst, J.J. Prohormone Convertase 1/3 Is Essential for Processing of the Glucose-dependent Insulinotropic Polypeptide Precursor. J. Biol. Chem. 2006, 281, 11050–11057.
- Usdin, T.B.; Mezey, E.; Button, D.C.; Brownstein, M.J.; Bonner, T.I. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993, 133, 2861–2870.
- Vilsbøll, T.; Krarup, T.; Deacon, C.F.; Madsbad, S.; Holst, J.J. Reduced Postprandial Concentrations of Intact Biologically Active Glucagon-Like Peptide 1 in Type 2 Diabetic Patients. Diabetes 2001, 50, 609–613.
- Brown, J.C.; Mutt, V.; Pederson, R.A. Further purification of a polypeptide demonstrating enterogastrone activity. J. Physiol. 1970, 209, 57–64.
- Heinrich, G.; Gros, P.; Habener, J.F. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. J. Biol. Chem. 1984, 259, 14082–14087.
- Vella, A. Effect of glucagon-like peptide1 (7-36) amide on glucose effectiveness and insulin action in people with type 2 diabetes. Diabetes 2000, 49, 611–617.
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181.
- Ward, W.K.; Bolgiano, D.C.; McKnight, B.; Halter, J.B.; Porte, D. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J. Clin. Investig. 1984, 74, 1318–1328.
- Meier, J.J.; Nauck, M.A. Is the Diminished Incretin Effect in Type 2 Diabetes Just an Epi-Phenomenon of Impaired β-Cell Function? Diabetes 2010, 59, 1117–1125.
- Stonehouse, A.H.; Holcombe, J.H.; Kendall, D.M. Management of Type 2 diabetes: The role of incretin mimetics. Expert Opin. Pharmacother. 2006, 7, 2095–2105.
- Nauck, M.A.; Heimesaat, M.M.; Orskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307.
- Kjems, L.L.; Holst, J.J.; Vølund, A.; Madsbad, S. The Influence of GLP-1 on Glucose-Stimulated Insulin Secretion: Effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003, 52, 380–386.
- Drucker, D.J.; Sherman, S.I.; Gorelick, F.S.; Bergenstal, R.M.; Sherwin, R.S.; Buse, J.B. Incretin-Based Therapies for the Treatment of Type 2 Diabetes: Evaluation of the Risks and Benefits. Diabetes Care 2010, 33, 428–433.
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705.
- Hansen, K.B.; Knop, F.K.; Holst, J.J.; Vilsbøll, T. Treatment of type 2 diabetes with glucagon-like peptide-1 receptor agonists. Int. J. Clin. Pract. 2009, 63, 1154–1160.
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962.
- American Diabetes Association Standards of Medical Care in Diabetes—2012. Diabetes Care 2012, 35 (Suppl. 1), S11–S63.
- Holst, J.J. From the Incretin Concept and the Discovery of GLP-1 to Today’s Diabetes Therapy. Front. Endocrinol. 2019, 10, 260.
- Prigeon, R.L.; Quddusi, S.; Paty, B.; D’Alessio, D.A. Suppression of endogenous glucose production by glucagon-like peptide1 independent of islet hormones: An extrapancreatic effect of an incretin hormone. Am. J. Physiol. 2003, 285, 701–707.
- Bernsmeier, C.; Meyer-Gerspach, A.C.; Blaser, L.S.; Jeker, L.; Steinert, R.E.; Heim, M.; Beglinger, C. Glucose-Induced Glucagon-Like Peptide 1 Secretion Is Deficient in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2014, 9, e87488.
- Yu, M.; Moreno, C.; Hoagland, K.M.; Dahly, A.; Ditter, K.; Mistry, M.; Roman, R.J. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J. Hypertens. 2003, 21, 1125–1135.
- Gutzwiller, J.P.; Tschopp, S.; Bock, A.; Zehnder, C.E.; Huber, A.R.; Kreyenbuehl, M.; Gutmann, H.; Drewe, J.; Henzen, C.; Goeke, B.; et al. Glucagon-Like Peptide 1 Induces Natriuresis in Healthy Subjects and in Insulin-Resistant Obese Men. J. Clin. Endocrinol. Metab. 2004, 89, 3055–3061.
- Turton, M.D.; O’Shea, D.; Gunn, I.; Beak, S.A.; Edwards, C.M.B.; Meeran, K.; Choi, S.J.; Taylor, G.M.; Heath, M.M.; Lambert, P.D.; et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72.
- Meeran, K.; O’Shea, D.; Edwards, C.M.B.; Turton, M.D.; Heath, M.M.; Gunn, I.; Abusnana, S.; Rossi, M.; Small, C.J.; Goldstone, A.P.; et al. Repeated Intracerebroventricular Administration of Glucagon-Like Peptide-1-(7-36) Amide or Exendin-(9-39) Alters Body Weight in the Rat. Endocrinology 1999, 140, 244–250.
- Abbott, C.R.; Monteiro, M.; Small, C.J.; Sajedi, A.; Smith, K.L.; Parkinson, J.R.; Ghatei, M.A.; Bloom, S.R. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005, 1044, 127–131.
- During, M.J.; Cao, L.; Zuzga, D.S.; Francis, J.S.; Fitzsimons, H.L.; Jiao, X.; Bland, R.J.; Klugmann, M.; Banks, W.A.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003, 9, 1173–1179.
- Perry, T.; Haughey, N.J.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Protection and Reversal of Excitotoxic Neuronal Damage by Glucagon-Like Peptide-1 and Exendin-4. J. Pharmacol. Exp. Ther. 2002, 302, 881–888.
- Nyberg, J.; Anderson, M.F.; Meister, B.; Alborn, A.M.; Ström, A.K.; Brederlau, A.; Illerskog, A.C.; Nilsson, O.; Kieffer, T.J.; Hietala, M.A.; et al. Glucose-Dependent Insulinotropic Polypeptide Is Expressed in Adult Hippocampus and Induces Progenitor Cell Proliferation. J. Neurosci. 2005, 25, 1816–1825.
- Salera, M.; Giacomoni, P.; Pironi, L.; Cornia, G.; Capelli, M.; Marini, A.; Benfenati, F.; Miglioli, M.; Barbara, L. Gastric Inhibitory Polypeptide Release after Oral Glucose: Relationship to Glucose Intolerance, Diabetes Mellitus, and Obesity. J. Clin. Endocrinol. Metab. 1982, 55, 329–336.
- Creutzfeldt, W.; Ebert, R.; Willms, B.; Frerichs, H.; Brown, J.C. Gastric inhibitory polypeptide (GIP) and insulin in obesity: Increased response to stimulation and defective feedback control of serum levels. Diabetologia 1978, 14, 15–24.
- Gault, V.A.; Irwin, N.; Green, B.D.; McCluskey, J.T.; Greer, B.; Bailey, C.J.; Harriott, P.; O’Harte, F.P.; Flatt, P.R. Chemical Ablation of Gastric Inhibitory Polypeptide Receptor Action by Daily (Pro3)GIP Administration Improves Glucose Tolerance and Ameliorates Insulin Resistance and Abnormalities of Islet Structure in Obesity-Related Diabetes. Diabetes 2005, 54, 2436–2446.
- Bollag, R.J.; Zhong, Q.; Phillips, P.; Min, L.; Zhong, L.; Cameron, R.; Mulloy, A.L.; Rasmussen, H.; Qin, F.; Ding, K.H.; et al. Osteoblast-Derived Cells Express Functional Glucose-Dependent Insulinotropic Peptide Receptors1. Endocrinology 2000, 141, 1228–1235.
- Zhong, Q.; Itokawa, T.; Sridhar, S.; Ding, K.H.; Xie, D.; Kang, B.; Bollag, W.B.; Bollag, R.J.; Hamrick, M.; Insogna, K.; et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am. J. Physiol. 2007, 292, 543–548.
- Fürnsinn, C.; Ebner, K.; Waldhäusl, W. Failure of GLP-1(7-36)amide to affect glycogenesis in rat skeletal muscle. Diabetologia 1995, 38, 864–867.
- Larsen, P.J.; Tang-Christensen, M.; Jessop, D.S. Central Administration of Glucagon-Like Peptide-1 Activates Hypothalamic Neuroendocrine Neurons in the Rat. Endocrinology 1997, 138, 4445–4455.
- Lacroix, A.; Bolté, E.; Tremblay, J.; Dupré, J.; Poitras, P.; Fournier, H.; Garon, J.; Garrel, D.; Bayard, F.; Taillefer, R.; et al. Gastric Inhibitory Polypeptide-Dependent Cortisol Hypersecretion—A New Cause of Cushing’s Syndrome. N. Engl. J. Med. 1992, 327, 974–980.
- Chaudhuri, A.; Ghanim, H.; Vora, M.; Sia, C.L.; Korzeniewski, K.; Dhindsa, S.; Makdissi, A.; Dandona, P. Exenatide Exerts a Potent Antiinflammatory Effect. J. Clin. Endocrinol. Metab. 2012, 97, 198–207.
- Seino, Y.; Yabe, D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J. Diabetes Investig. 2013, 4, 108–130.
- Kahles, F.; Meyer, C.; Möllmann, J.; Diebold, S.; Findeisen, H.M.; Lebherz, C.; Trautwein, C.; Koch, A.; Tacke, F.; Marx, N.; et al. GLP-1 Secretion Is Increased by Inflammatory Stimuli in an IL-6-Dependent Manner, Leading to Hyperinsulinemia and Blood Glucose Lowering. Diabetes 2014, 63, 3221–3229.
- Hopsu-Havu, V.K.; Glenner, G.G. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-beta-naphthylamide. Histochemie 1966, 7, 197–201.
- Sell, H.; Blüher, M.; Klöting, N.; Schlich, R.; Willems, M.; Ruppe, F.; Knoefel, W.T.; Dietrich, A.; Fielding, B.A.; Arner, P.; et al. Adipose Dipeptidyl Peptidase-4 and Obesity: Correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care 2013, 36, 4083–4090.
- Chen, X. Biochemical Properties of Recombinant Prolyl Dipeptidases DPP-IV and DPP8. Dipeptidyl Aminopeptidases 2006, 575, 27–32.
- Havre, P.A.; Abe, M.; Urasaki, Y.; Ohnuma, K.; Morimoto, C.; Dang, N.H. The role of CD26/dipeptidyl peptidase IV in cancer. Front. Biosci. 2008, 13, 1634–1645.
- Shah, Z.; Kampfrath, T.; Deiuliis, J.; Zhong, J.; Pineda, C.; Ying, Z.; Xu, X.; Lu, B.; Moffatt-Bruce, S.; Durairaj, R.; et al. Long-Term Dipeptidyl-Peptidase 4 Inhibition Reduces Atherosclerosis and Inflammation via Effects on Monocyte Recruitment and Chemotaxis. Circulation 2011, 124, 2338–2349.
- Aini, K.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Sata, M. Vildagliptin, a DPP-4 Inhibitor, Attenuates Endothelial Dysfunction and Atherogenesis in Nondiabetic Apolipoprotein E-Deficient Mice. Int. Heart J. 2019, 60, 1421–1429.
- Barnett, A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int. J. Clin. Pract. 2006, 60, 1454–1470.
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in diabetes. Front. Immunol. 2015, 6, 386.
- Boniol, M.; Franchi, M.; Bota, M.; Leclercq, A.; Guillaume, J.; van Damme, N.; Corrao, G.; Autier, P.; Boyle, P. Incretin-Based Therapies and the Short-term Risk of Pancreatic Cancer: Results from Two Retrospective Cohort Studies. Diabetes Care 2017, 41, 286–292.

