Statins are a cornerstone in the pharmacological prevention of cardiovascular disease and amongst the most commonly prescribed medications worldwide. Although generally well tolerated, a small subset of patients experience statin-related myotoxicity (SRM). SRM is heterogeneous in presentation; phenotypes include the relatively more common myalgias, infrequent myopathies, and rare rhabdomyolysis. Very rarely, statins induce an anti-HMGCR positive immune-mediated necrotizing myopathy. Diagnosing SRM in clinical practice can be challenging, particularly for mild SRM that is frequently due to alternative aetiologies and the nocebo effect. Nevertheless, SRM can directly harm patients and lead to statin discontinuation/non-adherence, which increases the risk of cardiovascular events. The aetiology of SRM has not been fully elucidated, but two inter-dependent mechanisms have been identified: increased systemic statin exposure, and disruption of muscle function. Several factors increase systemic statin exposure and predispose to SRM, including advanced age, concomitant medications, and the nonsynonymous variant, rs4149056, in SLCO1B1, which encodes the hepatic sinusoidal transporter, OATP1B1. Increased exposure of skeletal muscle to statins increases the risk of mitochondrial dysfunction, calcium signalling disruption, reduced prenylation, atrogin-1 mediated atrophy and pro-apoptotic signalling. SRM remains a challenge to the safe and effective use of statins, although consensus strategies to manage SRM have been proposed.
- statin
- muscle
- clinical presentation
- frequency
Statin-Related Myotoxicity (SRM) Clinical Presentation
Statin-related myotoxicity (SRM) constitutes the most commonly reported statin adverse event, comprising approximately two-thirds of all statin adverse events [68]. The most common muscular symptoms are pain, heaviness, stiffness and cramps with or without subjective weakness [58,69]. Symptoms involving leg muscles (thighs, calves) are most frequent, although back, neck, shoulder and generalised muscular symptoms have also been described [58,69]. Tendonitis-associated pain has been reported [58]. Approximately 40% of patients with SRM note a potential trigger; most commonly, unusual physical exertion or a new medication [58]. Muscular pains are intermittent in three quarters of SRM patients, and constant in one quarter [58].
SRM is most common during the first year of treatment [70] with a median time to onset of one month [51]; over 80% of patients report not experiencing similar symptoms before statin treatment [58]. The muscular symptoms in the majority of SRM cases (~70–80%) are sufficiently intense to disrupt everyday activities [58,69]; moreover they have been associated with reduced statin adherence and statin discontinuation, which can increase the risk of major adverse cardiovascular events (MACE). The rarer severe myopathies and rhabdomyolysis can lead directly to hospitalisation.
SRM Definitions
SRM is heterogeneous in presentation (Figure 1) and so case definitions vary between studies. Therefore, a recent effort has standardised nomenclature and classified SRM into seven distinct phenotypic categories [64]:
SRM 0 represents asymptomatic elevations in serum creatine kinase (CK) < 4 × the upper limit of normal (ULN);
SRM 1 and 2 are common myalgias (aches, cramps and/or weakness) with no (SRM 1) or minor CK elevations (< 4 × ULN, SRM 2);
SRM 3 represents increasingly infrequent myopathy with CK > 4 × but < 10 × ULN;
SRM 4 is severe myopathy with CK > 10 × but < 50 × ULN;
SRM 5 constitutes rare but potentially life-threatening rhabdomyolysis with either CK > 10 x ULN, muscle symptoms and renal impairment, or CK > 50 × ULN, and;
SRM 6 consists of very rare anti-3-hydroxy-3-methylglutaryl-Coenzyme A reductase (HMGCR) positive immune-mediated necrotizing myopathy, which persists despite statin cessation [64].
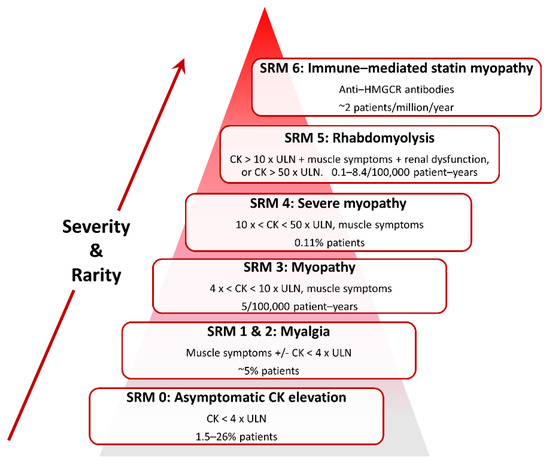
Figure 1. Classification of statin-related myotoxicity phenotypes.
Classification and estimated frequencies in Figure 1 are based on Alfirevic et al., 2014 [64], except the myalgia frequency which is from Parker et al., 2013 [65].
Whilst these categories will standardise research, they are perhaps less meaningful as diagnostic criteria in clinical practice. The National Lipid Association (NLA) defines statin intolerance as the “inability to tolerate at least two statins: one statin at the lowest starting daily dose and another statin at any daily dose, due to either objectionable symptoms (real or perceived) or abnormal laboratory determinations, which are temporally related to statin treatment and reversible upon statin discontinuation [66].” The European Atherosclerosis Society (EAS) states “the assessment of statin-associated muscle symptoms includes the nature of muscle symptoms, increased CK levels and their temporal association with initiation of therapy with statin, and statin therapy suspension and re-challenge [67].”
SRM Frequency
Amongst licensed statins, the frequency of SRM appears highest with simvastatin, followed by atorvastatin, and is lowest with fluvastatin [58]. However, the true incidence of SRM is uncertain, occurring in 1.5–5% of participants in randomized controlled trials (RCTs) [71], compared to ~10–33% in observational studies [61,72]. This variability is potentially attributable to a range of factors, including different myotoxicity definitions and follow up procedures, lead-in periods, inclusion of different patient groups, and treatment blinding [73]. There is consensus that statins increase the risk of severe myopathy and rhabdomyolysis [50]. Of note, cerivastatin was voluntarily withdrawn in 2001 because of 52 cases of fatal rhabdomyolysis [74]. However, the variability in reported SRM rates has sparked significant disagreement and controversy over the underlying benefit-risk profile of statins, particularly in patients thought to be at the lower end of the cardiovascular disease risk spectrum [75].
The greater difficulty lies in determining the aetiology of the commoner milder musculoskeletal symptoms, and in particular, whether they are attributable to a statin and/or concurrent condition(s) (e.g., viral illnesses). On the one hand, the frequency of muscle-related adverse events did not differ between patients on atorvastatin 10 mg daily or placebo in the large double-blind ASCOT-LLA RCT (n = 10,180), but became significantly more common in patients taking atorvastatin 10 mg daily (1.26% per annum) compared to placebo (1.00% per annum) in the subsequent open label non-blinded extension phase [76]. This observation was attributed to the nocebo effect. On the other hand, a six-month double-blind RCT conducted in 420 healthy volunteers administered atorvastatin 80 mg daily or placebo found increased myalgia amongst the subjects on atorvastatin compared to the placebo group (9.4% vs. 4.6%, respectively, p = 0.05) [65]. Moreover, N-of-1 (single-patient) placebo-controlled trials involving patients with a history of SRM have reported that ~30–40% experience subsequent muscle-related events only on statin and not placebo [77,78]. This suggests that the muscle symptoms experienced by a third of symptomatic patients are likely statin-induced, whilst the remainder are probably not. The challenge is how to distinguish patients with true SRM from those with myalgia due to other causes.
SRM Pathogenesis
Several SRM risk factors have been identified and mechanisms proposed, but there is not yet a unified pathophysiological understanding. Nevertheless, two inter-dependent mechanisms are implicated: 1. increased statin systemic exposure due to clinical and pharmacogenomic factors, which increase skeletal muscle exposure, and 2. intracellular skeletal myocyte entry and disruption of muscle function (Figure 2).
Several factors have been associated with increased systemic statin exposure including advanced age, low body mass index, chronic liver disease, higher statin dose, concomitant medications, and the nonsynonymous variant, rs4149056 (c.521T>C, pV174A), in solute carrier organic anion transporter family member 1B1 (SLCO1B1), which encodes the hepatic sinusoidal transporter, organic anion-transporting polypeptide 1B1 (OATP1B1). Administration of co-medications that inhibit cytochrome P450 3A (CYP3A) can substantially increase the plasma levels of the CYP3A4/5-substrate statins, atorvastatin, lovastatin, and simvastatin, and increase the risk of SRM. Importantly, the minor C allele of SLCO1B1 rs4149056 has been associated with significantly increased systemic exposure of all statins, except fluvastatin, and consistently with simvastatin-related myotoxicity. However, the evidence for a link between rs4149056 and SRM with the other statins is more variable and currently less compelling, although still subject to ongoing research.
Elevated systemic statin exposure plausibly increases intra-myocyte statin concentrations. Statin myocyte entry is likely facilitated by transporters, with statins being substrates for several sarcolemmal transporters. These include OATP2B1, multidrug resistance-associated protein (MRP) 1, MRP4, MRP5 and MCT4 (monocarboxylate transporter-4) [24,191]. It is also noteworthy that lipophilic statins (ATV, SVT) preferentially accumulate in skeletal muscle relative to hydrophilic statins (PVT, RVT) [192], which may help explain the greater myotoxicity of lipophilic statins [165].
Several mechanisms for statin-associated myocyte dysfunction have been proposed. For example, statins appear to potentiate exercise-related muscle injury. Pre-existing, but sometimes undiagnosed, neuromuscular disorders can increase susceptibility to SRM, including untreated hypothyroidism, and underlying metabolic myopathies. Thus, rare variants in several metabolic myopathy genes including CACNA1S, CPT2, LPIN1, PYGM and RYR1 have been identified in cases of myopathy/rhabdomyolysis following statin exposure. Other mechanisms of statin-associated myocyte dysfunction include impairment of mitochondrial function, reduced protein prenylation and potentially other downstream effects on the mevalonate pathway as a result of statin inhibition of HMGCR, genetic variation in pain perception, and immune-mediated mechanisms. The immune system has been implicated in conventional statin intolerance/myotoxicity via an association with leukocyte immunoglobulin-like receptor subfamily B member 5 (LILRB5) rs12975366 (D247G). Moreover, a strong association has been found between HLA-DRB1*11:01 and anti-HMGCR positive myopathy.
Further research is required, including stringent phenotyping of mild SRM through N-of-1 trials coupled to systems pharmacology omics- approaches to identify novel risk factors of SRM and provide further mechanistic understanding of the pathogenesis of SRM.
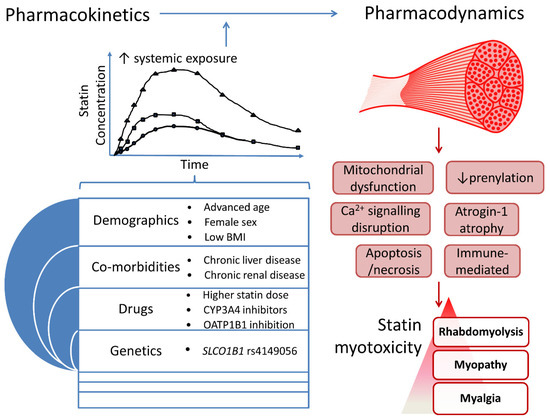
Figure 2. An integrated overview of processes implicated in statin myotoxicity.
This entry is adapted from the peer-reviewed paper 10.3390/jcm9010022
