In idiopathic pulmonary fibrosis (IPF), several factors may have a negative impact on the nutritional status, including an increased respiratory muscles load, release of inflammation mediators, the coexistence of hypoxemia, and physical inactivity. Nutritional abnormalities also have an impact on IPF clinical outcomes. Given the relevance of nutritional status in IPF patients, we sought to focus on some critical issues, highlighting what is known and what should be further learned about these issues. We revised scientific literature published between 1995 and August 2019 by searching on Medline/PubMed and EMBASE databases including observational and interventional studies. We conducted a narrative review on nutritional assessment in IPF, underlining the importance of nutritional evaluation not only in the diagnostic process, but also during follow-up. We also highlighted the need to keep a high level of attention on cardiovascular comorbidities. We also focused on current clinical treatment in IPF with Nintedanib and Pirfenidone and management of gastrointestinal adverse events, such as diarrhea, induced by these antifibrotic drugs. Finally, we concentrated on the importance of pulmonary rehabilitation program, including nutritional assessment, education and behavioral change, and psychological support among its essential components. More attention should be devoted to the assessment of the undernutrition and overnutrition, as well as of muscle strength and physical performance in IPF patients, taking also into account that an adequate clinical management of gastrointestinal complications makes IPF drug treatments more feasible.
- idiopathic pulmonary fibrosis
- nutrition abnormalities
- nutritional assessment
- rehabilitation
In recent years, nutritional status has increasingly gained attention in the evaluation of patients with chronic respiratory diseases since their clinical course is often characterized by progressive weight loss and reduction of muscle mass [1–3].
Idiopathic pulmonary fibrosis (IPF) is a fibrosing interstitial lung disease of unknown etiology characterized by rapid progression and poor prognosis [4,5].
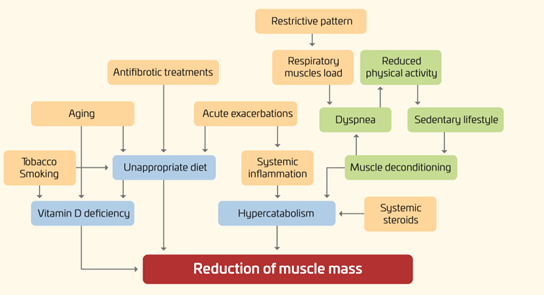
As shown in Figure 1, in IPF, several factors may have a negative impact on the nutritional status of IPF patients, including an increased respiratory muscles load, release of inflammation mediators, hypoxemia, and physical inactivity [6–9].
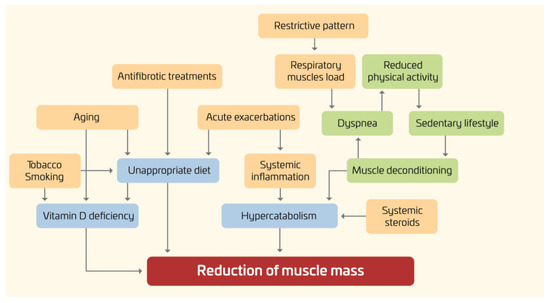
Figure 1. Nutritional disorders in IPF: pathogenesis.
Furthermore, given the rapidly progressive nature of the disease, with a median survival time of only two to five years, all these factors often develop and worsen in a short period of time.
It is worth noting that not only may the “downward spiral” of the underlying pulmonary disease affect the nutritional status, but also nutritional abnormalities may have an impact on clinical outcomes. For instance, lower body mass index (BMI) and body weight loss have been associated with increased mortality [10–12]. Conversely, the effects of obesity and metabolic syndrome are less known.
Given the relevance of evaluating nutritional status in IPF patients, a topic which remains still largely understudied, we sought to focus on some critical issues, highlighting what is known and what should be further learned about these issues.
Assessment of Nutritional Status in IPF Patients
Specific nutritional counseling and dietary advices for IPF patients are not yet available, with the exception of dietary recommendations to reduce the side effects of antifibrotic treatments (see the following sections). The implementation of a nutrition care process may be useful especially in those patients with IPF who are at risk or already malnourished [13]. According to the ESPEN guidelines [13], malnutrition (i.e., undernutrition) can be defined as “a state resulting from lack of intake or uptake of nutrition that leads to altered body composition (decreased fat free mass) and body cell mass leading to diminished physical and mental function and impaired clinical outcome from disease”. Disease-related malnutrition can arise from a number of reasons, for instance the inability to eat (for instance, as a result of anorexia, nausea, and vomiting), impaired absorption, altered metabolism, and hypercatabolism. The diagnosis of malnutrition (undernutrition) may be reached according to international criteria [13] or based on authors’ choices. The term overnutrition includes both overweight and obesity.
From a practical point of view, nutritional status may be assessed by evaluating nutrient intakes, energy expenditure, body composition, laboratory data, and body functions. Overall, only a few studies (in most cases, cross-sectional) have so far evaluated the nutritional status of IPF patients.
To the best of our knowledge, there are no consistent results available on nutrient intakes in IPF. Two studies carried out in Japan [14,15] showed that the consumption of fruit was associated with a reduced risk of IPF, whereas the opposite was true for meat and saturated fatty acids.
Main anthropometric data were reported in many papers. Mean BMI was around 26–28 kg/m2 in most studies from Western Countries [8,10,16–21] and lower (23–24 kg/m2) in those carried out in Japan and South Korea [11,22–25].
Thus, a high proportion of overweight/obese patients (overnutrition) may be expected [17,21]. As a matter of fact, in terms of phenotype, the prevalence of obese patients has been reported in two studies to be 34% and 46% [10,21]. On the other hand, the prevalence of underweight patients was found low (4%), but of clinical significance, in a recent study on French IPF patients [18], whereas it was substantially higher (>20%) in patients recruited in Japan [24]. As far as body composition is concerned, two studies found low fat-free mass (strongly suggesting malnutrition) in a consistent proportion of IPF patients [18,24]. The study by Jouneau et al. also reported low values of mid-arm circumference in nearly one-third of cases [18]. Interestingly, a reduction of pectoralis muscle area (no difference in BMI) was specifically observed in IPF patients with frailty syndrome [21]. Finally, it should be stressed that fat-free mass is also a significant independent predictor of survival in IPF patients [24]. With regard to vitamin status, a recent study showed that patients with IPF exhibited low serum vitamin D concentrations and such deficiency correlated with all-cause mortality, suggesting the role of vitamin D as prognostic factor and therapeutic target [26]. Overall, it should be noted that there are no studies identifying malnutrition and/or sarcopenia, as formal diagnoses, using to international criteria such as those proposed by ESPEN [13] or the 2019 EWGSOP Consensus [27], respectively.
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27]
References
- Dominika Mękal; Anna Doboszyńska; E. Kądalska; E. Swietlik; L. Rudnicka; Nutritional Status in Chronic Obstructive Pulmonary Disease and Systemic Sclerosis: Two Systemic Diseases Involving the Respiratory System. Advances in Experimental Medicine and Biology 2014, 840, 45-49, 10.1007/5584_2014_19.
- Andre P. Hitzl; Rudolf A. Jörres; Frank Heinemann; Michael Pfeifer; Stephan Budweiser; Nutritional status in patients with chronic respiratory failure receiving home mechanical ventilation: Impact on survival. Clinical Nutrition 2010, 29, 65-71, 10.1016/j.clnu.2009.08.002.
- Annemie M.W.J. Schols; Ivone M. Ferreira; Frits M. Franssen; Harry R. Gosker; Wim Janssens; Maurizio Muscaritoli; Christophe Pison; Maureen Rutten-Van Mölken; Frode Slinde; Michael C. Steiner; Ruzena Tkacova; Sally J. Singh; Nutritional assessment and therapy in COPD: a European Respiratory Society statement. European Respiratory Journal 2014, 44, 1504-1520, 10.1183/09031936.00070914.
- Giovanni Ferrara; Lisa Carlson; Andreas Palm; Jonas Einarsson; Cecilia Olivesten; Magnus Sköld; for the Swedish Idiopathic Pulmonary Fibrosis Registry Group; Idiopathic pulmonary fibrosis in Sweden: report from the first year of activity of the Swedish IPF-Registry. European Clinical Respiratory Journal 2016, 3, 31090, 10.3402/ecrj.v3.31090.
- M.L. Rosado De Christenson; An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Yearbook of Diagnostic Radiology 2012, 2012, 29-30, 10.1016/j.yrad.2012.03.015.
- Chiara Scelfo; Antonella Caminati; Sergio Harari; Recent advances in managing idiopathic pulmonary fibrosis. F1000Research 2017, 6, 2052, 10.12688/f1000research.10720.1.
- Ayadi Nouha; Feki Walid; Marrakchi Rim; Mkaouar Najla; Ketata Wajdi; Bahloul Najla; Hajer Ayadi; Msaad Sameh; Yangui Ilhem; W.K. Rekik; Jamoussi Kamel; Kammoun Samy; Assessment of nutritional status in patients with idiopathic pulmonary fibrosis. Clinical Problems 2016, 48, , 10.1183/13993003.congress-2016.pa3703.
- Sabina Guler; Seo Am Hur; Scott A. Lear; Pat Camp; Christopher J. Ryerson; Body composition, muscle function, and physical performance in fibrotic interstitial lung disease: a prospective cohort study. Respiratory Research 2019, 20, 56, 10.1186/s12931-019-1019-9.
- Sayed Esmaeil Mousavi; Heresh Amini; Pouria Heydarpour; Fatemeh Amini Chermahini; Lode Godderis; Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms. Environment International 2019, 122, 67-90, 10.1016/j.envint.2018.11.052.
- Mazen Alakhras; Paul A. Decker; Hassan F. Nadrous; Maria Collazo-Clavell; Jay H. Ryu; Body Mass Index and Mortality in Patients With Idiopathic Pulmonary Fibrosis. Chest 2007, 131, 1448-1453, 10.1378/chest.06-2784.
- Yoshinari Nakatsuka; Tomohiro Handa; Maria Kokosi; Kiminobu Tanizawa; Silvia Puglisi; Joseph Jacob; Akihiko Sokai; Kohei Ikezoe; Kumiko T. Kanatani; Takeshi Kubo; Hiromi Tomioka; Yoshio Taguchi; S Nagai; Kazuo Chin; Michiaki Mishima; Athol U. Wells; Toyohiro Hirai; The Clinical Significance of Body Weight Loss in Idiopathic Pulmonary Fibrosis Patients. Respiration 2018, 96, 1-10, 10.1159/000490355.
- Janelle Pugashetti; Julia Graham; Noelle Boctor; Cesar Mendez; Elena Foster; Maya Juarez; Richart Harper; Brian Morrissey; Michael Kadoch; Justin M. Oldham; Weight loss as a predictor of mortality in patients with interstitial lung disease.. European Respiratory Journal 2018, 52, 1801289, 10.1183/13993003.01289-2018.
- T. Cederholm; Rocco Barazzoni; P.D. Austin; Peter E. Ballmer; Gianni Biolo; Stephan Bischoff; Charlene Compher; M. Isabel T. D. Correia; T. Higashiguchi; Mette Holst; Gordon L. Jensen; Ainsley Malone; Alessio Molfino; Ibolya B. Nyulasi; M. Pirlich; Elisabet Rothenberg; Karin Schindler; Stéphane M. Schneider; M.A.E. De Van Der Schueren; Cornel Sieber; Luzia Valentini; Jian-Chun Yu; André Van Gossum; Pierre Singer; ESPEN guidelines on definitions and terminology of clinical nutrition.. Clinical Nutrition 2016, 36, 49-64, 10.1016/j.clnu.2016.09.004.
- Yoshihiro Miyake; S Sasaki; T Yokoyama; K Chida; A Azuma; T Suda; S Kudoh; N Sakamoto; K Okamoto; G Kobashi; M Washio; Y Inaba; H Tanaka; Dietary fat and meat intake and idiopathic pulmonary fibrosis: a case-control study in Japan.. The International Journal of Tuberculosis and Lung Disease 2006, 10, , null.
- Yoshihiro Miyake; Satoshi Sasaki; Tetsuji Yokoyama; Kingo Chida; Arata Azuma; Takafumi Suda; Shoji Kudoh; N Sakamoto; Kazushi Okamoto; Gen Kobashi; Masakazu Washio; Yutaka Inaba; Heizo Tanaka; Vegetable, Fruit, and Cereal Intake and Risk of Idiopathic Pulmonary Fibrosis in Japan. Annals of Nutrition and Metabolism 2004, 48, 390-397, 10.1159/000082465.
- Marco Mura; Maria A. Porretta; Elena Bargagli; Gianluigi Sergiacomi; Maurizio Zompatori; Nicola Sverzellati; Amedeo Taglieri; Fabrizio Mezzasalma; Paola Rottoli; Cesare Saltini; Paola Rogliani; Predicting survival in newly diagnosed idiopathic pulmonary fibrosis: a 3-year prospective study. European Respiratory Journal 2012, 40, 101-109, 10.1183/09031936.00106011.
- Claire M. Nolan; Matthew Maddocks; Toby M Maher; Winston Banya; Suhani Patel; Ruth E. Barker; Sarah E. Jones; Peter M. George; Paul Cullinan; William D.-C. Man; Gait speed and prognosis in patients with idiopathic pulmonary fibrosis: a prospective cohort study. European Respiratory Journal 2019, 53, 1801186, 10.1183/13993003.01186-2018.
- Jouneau, S.; Kerjouan, M.; Rousseau, C.; Lederlin, M.; Llamas-Guttierez, F.; De Latour, B.; Guillot, S.; Vernhet, L.; Desrues, B.; Thibault, R. What are the best indicators to assess malnutrition in idiopathic pulmonary fibrosis patients? A cross-sectional study in a referral center. Nutrition 2019, 62, 115–121.
- Stéphane Jouneau; Anne-Sophie Gamez; Julie Traclet; Hilario Nunes; Sylvain Marchand-Adam; Romain Kessler; Dominique Israël-Biet; Raphael Borie; Indiana Strombom; Astrid Scalori; Bruno Crestani; Minique Valeyre; Vincent Cottin; A 2-Year Observational Study in Patients Suffering from Idiopathic Pulmonary Fibrosis and Treated with Pirfenidone: A French Ancillary Study of PASSPORT.. Respiration 2019, 98, 19-28, 10.1159/000496735.
- Claire Marie Nolan; Matthew Maddocks; Toby M. Maher; Jane L. Canavan; Sarah E. Jones; Ruth E. Barker; Suhani Patel; Joseph Jacob; Paul Cullinan; William D.-C. Man; Phenotypic characteristics associated with slow gait speed in idiopathic pulmonary fibrosis. Respirology 2017, 23, 498-506, 10.1111/resp.13213.
- Jamie S. Sheth; Meng Xia; Susan Murray; Carlos H. Martinez; Catherine A. Meldrum; Elizabeth A. Belloli; Margaret L Salisbury; Eric S. White; Colin H. Holtze; Kevin R. Flaherty; Frailty and geriatric conditions in older patients with idiopathic pulmonary fibrosis.. Respiratory Medicine 2019, 148, 6-12, 10.1016/j.rmed.2019.01.008.
- Brett Ley; Harold R. Collard; Talmadge E. King; Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. American Journal of Respiratory and Critical Care Medicine 2011, 183, 431-440, 10.1164/rccm.201006-0894ci.
- Akira Morino; Hiroki Takahashi; Hirofumi Chiba; Sumio Ishiai; Daily physical activity affects exercise capacity in patients with idiopathic pulmonary fibrosis. Journal of Physical Therapy Science 2017, 29, 1323-1328, 10.1589/jpts.29.1323.
- Osamu Nishiyama; Ryo Yamazaki; Hiroyuki Sano; Takashi Iwanaga; Yuji Higashimoto; Hiroaki Kume; Yuji Tohda; Fat-free mass index predicts survival in patients with idiopathic pulmonary fibrosis. Respirology 2016, 22, 480-485, 10.1111/resp.12941.
- Satoshi Ikeda; Akimasa Sekine; Tomohisa Baba; Takuma Katano; Erina Tabata; Ryota Shintani; Shinko Sadoyama; Hideaki Yamakawa; Tsuneyuki Oda; Ryo Okuda; Hideya Kitamura; Tae Iwasawa; Tamiko Takemura; Takashi Ogura; Negative impact of anorexia and weight loss during prior pirfenidone administration on subsequent nintedanib treatment in patients with idiopathic pulmonary fibrosis. BMC Pulmonary Medicine 2019, 19, 78, 10.1186/s12890-019-0841-7.
- Vassilis Aidinis; Faculty Opinions recommendation of Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs.. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2008, null, , 10.3410/f.1104852.560937.
- N.I. Dzerovych; 2019 European recommendations on sarcopenia diagnosis. PAIN, JOINTS, SPINE 2019, 9, 257-261, 10.22141/2224-1507.9.4.2019.191925.