Anodic oxides is part of energy conversion and storage devices.
- Anodic Oxides
- Energy
1. Introduction
This entry presents studies that bring applied technological aspects that are being developed or are already in the implementation stage and does not present an in-depth discussion regarding anodic oxide materials’ synthetic methodology. The fabrication of anodic oxides from anodization of metallic substrates offers several advantages that can impact the technology economically in biological, environmental, and energy fields. The anodic oxidation is a facile, low-cost, and scalable method to obtain nanostructured porous oxide films with large surface areas by strict control of the morphological and structural properties, such as tube length, pore size, film thickness, and rigid adhesion to the substrate [1,2,3][1][2][3]. These features make the anodic films, such as the popular TiO2 nanotubes (TiO2-NT) and anodic aluminum oxide (AAO), attractive for the development of a wide range of technologies, including drug delivery systems [4], implants [5], sensors [6], filtration membranes [7], photocatalysts for pollutant degradation [8], energy conversion [9,10,11][9][10][11] and energy-storage systems [12[12][13],13], as illustrated in Scheme 1.
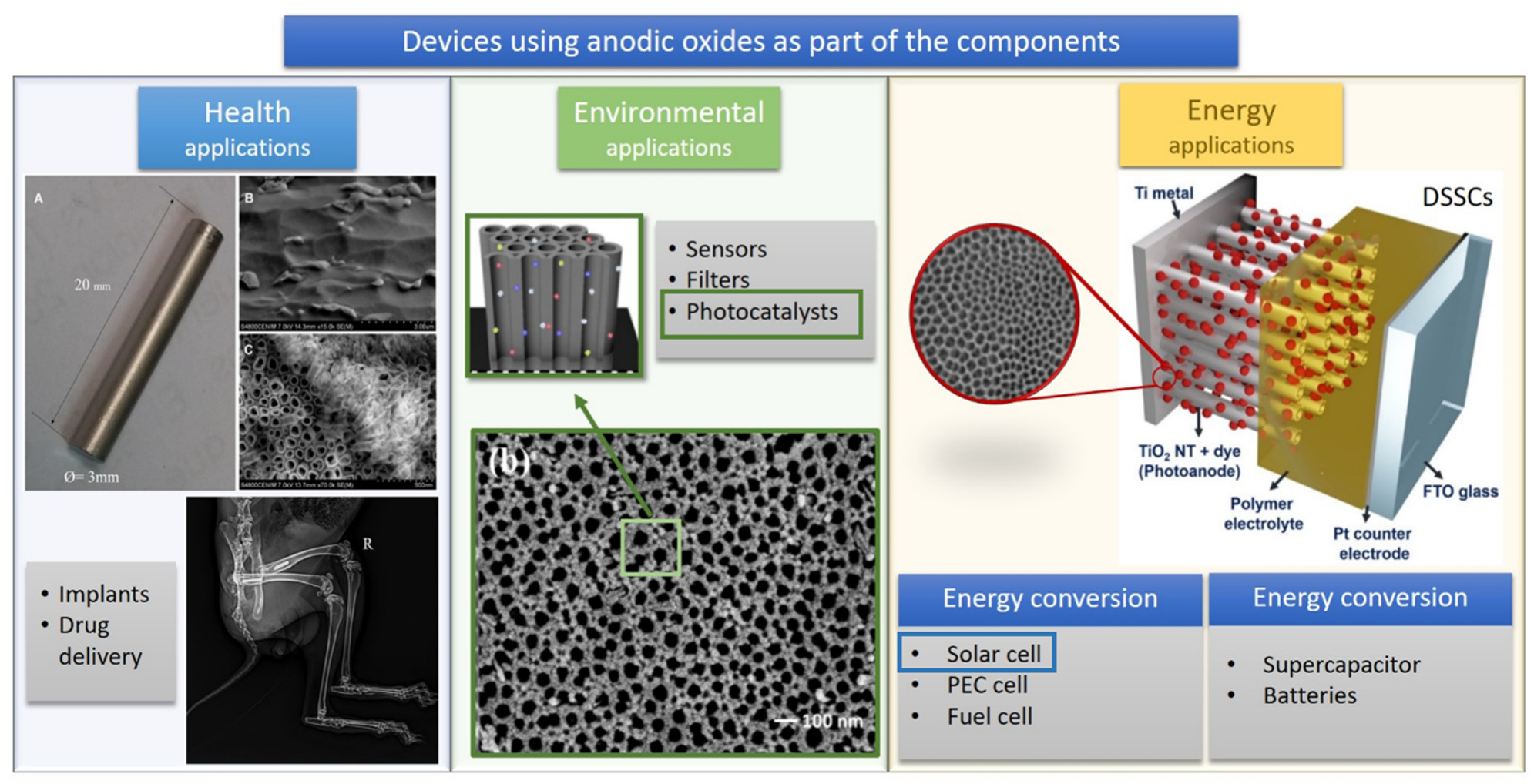
In the context of global sustainable strategies used to minimize the problems caused by industrialization and population growth, scientists have made significant efforts to develop advanced materials and systems based on clean processes that exploit renewable energy sources. In this sense, solar energy, a “green” resource, is essential as a substitute for conventional and non-renewable energy sources due to its abundance, availability, and direct harvest. The solar light can be directly converted into electric energy by photovoltaic (PV) devices such as solar cells. On the other hand, the photoelectrochemical (PEC) devices operate using solar energy or another light source to produce hydrogen gas from water molecules. Considered as a green fuel, hydrogen can be stored or converted into electric energy in fuel cells.
Therefore, PV, PEC, and hybrid PV–PEC systems are considered attractive technologies for sustainable production of electric energy and renewable fuel. One of the main challenges in this sector is to improve the efficiency and performance of these devices by the fabrication of stable photoelectrodes with high photoconversion rates. In general, TiO2-NT is the preferred anodic oxide explored in dye-sensitized solar cells and PEC water-splitting systems [10,17,18,19][10][17][18][19]. Due to its photocatalytic properties, chemical stability, and unidirectional orientation, this semiconductor is frequently used as the photoanode; exceptions include reports of WO3 [20,21][20][21] and Fe2O3 [22]. The use of copper oxides as photocathodes is emerging [23,24][23][24]. The utilization of AAO as a template or scaffold is also addressed [2,25,26][2][25][26].
Considering devices that operate essentially via electrochemical processes, the most promising sustainable systems are the fuel cell (for energy conversion) and the supercapacitors and rechargeable batteries (for energy storage). In these applications, TiO2-NT is also the widely explored anodic oxide, but in this case, the properties of interest are its chemical stability, low-cost, and high surface area, despite its low conductivity [27,28][27][28]. In fuel cells, this oxide is employed as support for the catalyst in both anodes [29] and cathodes [30] to increase the electrodes’ stability. In supercapacitors, the excellent adhesion between anodic oxide and metal is advantageous for the fabrication of electrodes without a binder agent, reducing the synthesis steps [31]. In rechargeable batteries, large surface areas with open frameworks for insertion of ions are the aim. Additionally, the low volume expansion during the Li+ insertion/extraction is an advantage that can increase the stability and safety of the Li-ion battery [32]. Besides TiO2, the interest in the properties of other nanostructured anodic films for energy storage systems, such as Cu(OH)2, NiO, WO3, SnO, and Ta2O5, has been increasing in the field.
2. General Aspects of Anodic Oxide Synthesis for Energy Applications
Nanostructured anodic oxides are an excellent alternative for developing energy devices’ components due to a series of specific advantages present in each energy application. However, we can summarize the general advantages of their utilization as follows:
- The anodic oxides are generally synthesized in environmentally friendly experimental conditions (mild temperature synthesis and low toxicity substances applied);
- They offer facile control of synthesis parameters, such as domain morphology, composition, and structure with the potential to anchor specific catalyst substances to use them as anode or cathode based-materials;
- The materials present a high surface area per volume;
- They allow facile modulation of nanostructure architecture to enhance ion transport;
- The mostly anodic oxides are chemically stable;
- In most cases, they offer excellent adhesion between different layers, avoiding binder agents.
The synthetic routes employed to fabricate the anodic oxides generally involve the following steps: (i) pre-treatment of the substrate, (ii) anodization, (iii) heat treatment, and (iv) surface modification if necessary. This section depicts the common aspects of the syntheses of TiO2, Al2O3, WO3, Fe2O3, ZnO, CuxO, ZrO2, NiO, SnO, Nb2O5, and Ta2O5, which include the anodization procedure to grow the anodic oxides, followed by heat treatment procedure for oxide modification or crystallization.
The pre-treatment steps (cleaning, polishing, pre-annealing procedures) will not be described here because they can vary according to the metal and purpose. For this, we recommend consulting the original papers for details. Furthermore, since some functionalities were designed to attend to the specific properties of the device´s materials, the additional steps employed for surface modification of the oxide will be described in the following sections with each material’s application description.
The anodization consists of an oxidation process of a metallic substrate (Mo → Mz+ + ze−) working as the anode of an electrochemical reactor in a proper electrolyte. The resulting anodic oxide film grown over the metal substrate can form a rigid compact layer or a nanostructured layer. The former case refers to barrier-type oxide films [2]. The latter case involves the formation of nanostructured oxide films with different morphologies, such as nanopores, nanotubes, nanowires, nanorods, nanopetals, and other nanogeometries [1,2,3,41,42][1][2][3][33][34]. The type of oxide formed depends on the experimental conditions. The main parameters that must be considered to control the morphology and composition of the anodic film during the anodization step are:
- Substrate: composition, purity, rugosity, and surface defects.
- Electrolyte: composition, temperature, and stirring.
- Electrical parameters: galvanostatic, potentiostatic, potentiodynamic, pulsed, or hybrid methods.
- Synthetic route: one-step, two-step or multi-step anodization.
- Anodizing time.
The mechanisms of growth of anodic oxide nanostructures and the influences of anodizing conditions on the oxide film’s final properties are well-known in the scientific literature, with many papers devoted to these topics having been published in the last decades [1,2,3,43,44,45][1][2][3][35][36][37]. In general, to form a nanostructured morphology with a large surface area, the anodization must be performed in an electrolyte in which the formed oxide is partially soluble, for instance, oxalate media to produce nanoporous alumina [2,45][2][37] or fluoride ions to grown nanotubes of TiO2 or ZrO2 [1,46,47][1][38][39]. The pore or nanotube diameter can be controlled by the applied potential and temperature, whereas the oxide layer’s thickness can be tuned by the anodizing time [48,49][40][41]. The anodizing time can vary from a few minutes to several hours depending on the layer’s desired thickness, usually obtained in the micrometer range [2,49,50][2][41][42]. Polishing procedures and the use of high-purity substrates tend to increase the orderly arrangement of the nanostructure. The two-step anodization method can be applied to the production of highly ordered nanotube/nanopore arrays [2,3][2][3]. This procedure uses an initial anodization step, followed by removing the oxide layer and a subsequent second anodization step over the nanotextured substrate.
On the other hand, the oxide’s microstructure and degree of crystallization are controlled by post-treatments annealing procedures in air or under a suitable gas atmosphere. The composition of the oxide surface can be also be tuned in this step. The anodization of Cu in alkaline solutions, for instance, leads to the formation of copper hydroxides that can be converted into CuO or Cu2O species after an annealing step [42][34]. Kang et al. [51][43] utilized a CO atmosphere to convert anodized WO3 into WC for a cathode in PEC applications.
The morphologies, compositions, and microstructures of the anodic films are usually analyzed by field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), transmission electron microscopy (TEM), and X-ray photoelectron microscopy (XPS) techniques. The electronic properties are commonly evaluated by diffuse reflectance spectroscopy (DRS) and electrochemical impedance spectroscopy (EIS).
The usual procedure adopted to form the nanotube arrays of TiO2 for energy applications is the conventional potentiostatic anodization of high-purity Ti foils in organic media containing fluoride ions plus a small amount of water. NH4F and H2O dissolved in ethylene glycol (EG) have been the preferred formulae for electrolyte composition [52,53,54,55,56,57][44][45][46][47][48][49]. Other studies substituted EG with glycerol and HF by NaF [11,58][11][50]. Aqueous solutions, such as CH3COOH + HF [51][43] and H3PO4 + NH4F [59][51], were also reported. It is possible to obtain nanotube arrays with a diameter ranging from 40 to 160 nm while applying potentials in the 10–60 V range for anodization times varying from 20 min to 24 h [29,49,59,60][29][41][51][52]. The high surface area provided a larger number of active sites for the photo and electrochemical processes. In some cases, two-step anodization was carried out to obtain a high-ordered nanotube array [18,53,61][18][45][53]. The syntheses were usually performed at room temperature, but an exception includes the anodization performed at 55 °C for the fabrication of TiO2-NT bioanodes in microbial fuel cell devices [11,62][11][54].
In those applications requiring a transparent conductive substrate, like in solar cells, the TiO2-NT membrane was detached from the Ti metal after anodizing via ultrasonication and transferred to the fluorine-doped Tin Oxide (FTO) glass, using a TiO2 paste to binding the layers [18,53][18][45].
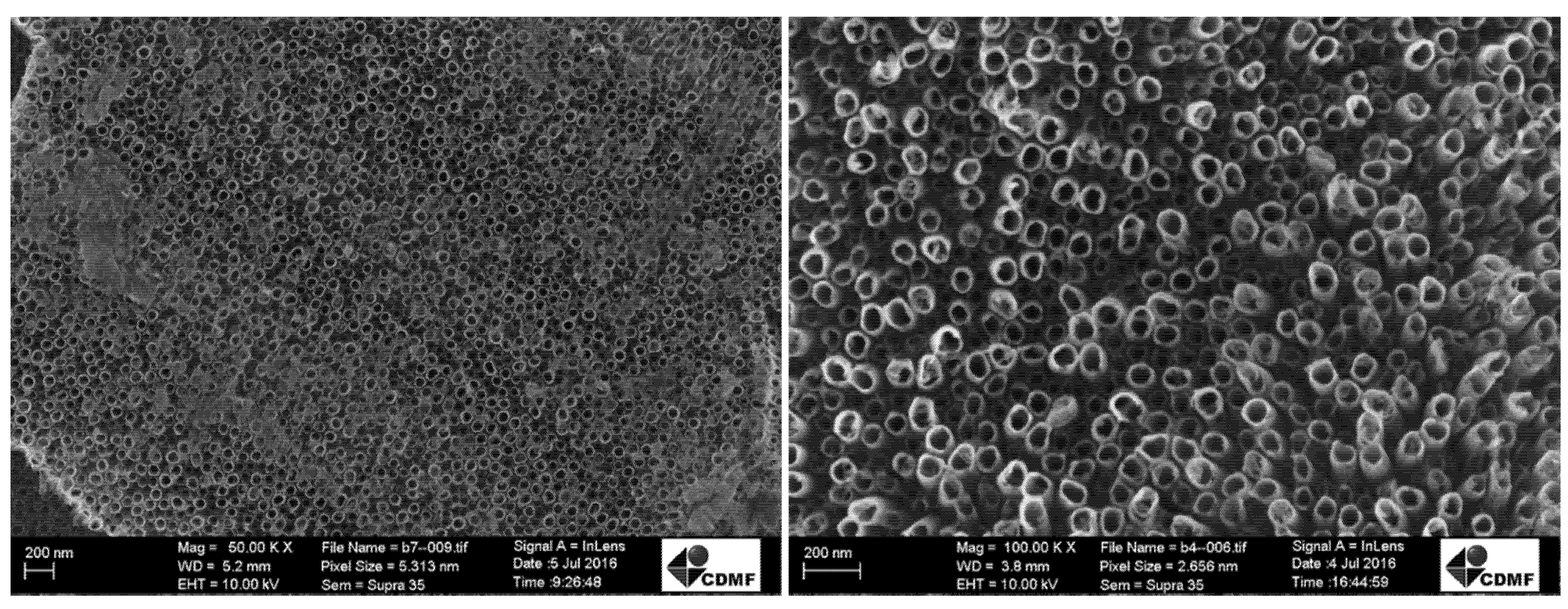
Figure 1 depicts a surface micrograph obtained by FESEM of a TiO2-NT sample prepared via anodization of Ti foil at 25 V for 90 min in ethylene glycol containing 10% wt. water and 0.75% wt. NH4F, after annealing at 450 °C for 2 h. The nanotubes exhibited a pore diameter in the 50–70 nm range with approximately 10 nm of wall thickness under this condition.
References
- Smith, Y.R.; Ray, R.S.; Carlson, K.; Sarma, B.; Misra, M. Self-Ordered Titanium Dioxide Nanotube Arrays: Anodic Synthesis and Their Photo/Electro-Catalytic Applications. Materials 2013, 6, 2892–2957.
- Trivinho-Strixino, F.; Santos, J.S.; Souza Sikora, M. 3-Electrochemical Synthesis of Nanostructured Materials. In Nanostructures; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; William Andrew Publishing: Park Ridge, NJ, USA, 2017; pp. 53–103.
- Sulka, G.D. Highly Ordered Anodic Porous Alumina Formation by Self-Organized Anodizing. In Nanostructured Materials in Electrochemistry; Eftekhari, A., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–116.
- Wang, Q.; Huang, J.Y.; Li, H.Q.; Zhao, A.Z.; Wang, Y.; Zhang, K.Q.; Sun, H.T.; Lai, Y.K. Recent advances on smart TiO2 nanotube platforms for sustainable drug delivery applications. Int. J. Nanomed. 2017, 12, 151–165.
- Awad, N.K.; Edwards, S.L.; Morsi, Y.S. A review of TiO2 NTs on Ti metal: Electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C 2017, 76, 1401–1412.
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous Anodic Alumina: A Versatile Platform for Optical Biosensors. Materials 2014, 7, 4297–4320.
- Barzegar, S.; Absalan, G.; Moradi, M.; Behaein, S. Constructing geometrically-ordered alumina nanoporous filters and alumina nanowire arrays by using ultrahigh voltage two step anodization. Phys. E Low Dimens. Syst. Nanostruct. 2020, 117, 113789.
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A Review of Photocatalysis using Self-organized TiO2 Nanotubes and Other Ordered Oxide Nanostructures. Small 2012, 8, 3073–3103.
- Yi, Z.; Zeng, Y.; Wu, H.; Chen, X.; Fan, Y.; Yang, H.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. Synthesis, surface properties, crystal structure and dye-sensitized solar cell performance of TiO2 nanotube arrays anodized under different parameters. Results Phys. 2019, 15, 102609.
- Ampelli, C.; Tavella, F.; Perathoner, S.; Centi, G. Engineering of photoanodes based on ordered TiO2-nanotube arrays in solar photo-electrocatalytic (PECa) cells. Chem. Eng. J. 2017, 320, 352–362.
- Feng, H.; Liang, Y.; Guo, K.; Chen, W.; Shen, D.; Huang, L.; Zhou, Y.; Wang, M.; Long, Y. TiO2 Nanotube Arrays Modified Titanium: A Stable, Scalable, and Cost-Effective Bioanode for Microbial Fuel Cells. Environ. Sci. Technol. Lett. 2016, 3, 420–424.
- Zhang, M.-M.; Chen, J.-Y.; Li, H.; Wang, C.-R. Recent progress in Li-ion batteries with TiO2 nanotube anodes grown by electrochemical anodization. Rare Met. 2020.
- Auer, A.; Kunze-Liebhäuser, J. Recent Progress in Understanding Ion Storage in Self-Organized Anodic TiO2 Nanotubes. Small Methods 2019, 3, 1800385.
- Auñón, Á.; Esteban, J.; Doadrio, A.L.; Boiza-Sánchez, M.; Mediero, A.; Eguibar-Blázquez, D.; Cordero-Ampuero, J.; Conde, A.; Arenas, M.-Á.; de-Damborenea, J.-J.; et al. Staphylococcus aureus Prosthetic Joint Infection Is Prevented by a Fluorine- and Phosphorus-Doped Nanostructured Ti–6Al–4V Alloy Loaded With Gentamicin and Vancomycin. J. Orthop. Res. 2020, 38, 588–597.
- Zhang, J.; Yang, C.; Li, S.; Xi, Y.; Cai, C.; Liu, W.; Golosov, D.; Zavadski, S.; Melnikov, S. Preparation of Fe3+ Doped High-Ordered TiO2 Nanotubes Arrays with Visible Photocatalytic Activities. Nanomaterials 2020, 10, 2107.
- Lee, A.R.; Kim, J.-Y. Highly Ordered TiO2 Nanotube Electrodes for Efficient Quasi-Solid-State Dye-Sensitized Solar Cells. Energies 2020, 13, 6100.
- Xiao, B.-C.; Lin, L.-Y. Substrate Diameter-Dependent Photovoltaic Performance of Flexible Fiber-Type Dye-Sensitized Solar Cells with TiO2 Nanoparticle/TiO2 Nanotube Array Photoanodes. Nanomaterials 2020, 10, 13.
- Rezaei, B.; Mohammadi, I.; Ensafi, A.A.; Momeni, M.M. Enhanced efficiency of DSSC through AC-electrophoretic hybridization of TiO2 nanoparticle and nanotube. Electrochim. Acta 2017, 247, 410–419.
- Stoll, T.; Zafeiropoulos, G.; Tsampas, M.N. Solar fuel production in a novel polymeric electrolyte membrane photoelectrochemical (PEM-PEC) cell with a web of titania nanotube arrays as photoanode and gaseous reactants. Int. J. Hydrogen Energy 2016, 41, 17807–17817.
- Li, L.; Zhao, X.; Pan, D.; Li, G. Nanotube array-like WO3/W photoanode fabricated by electrochemical anodization for photoelectrocatalytic overall water splitting. Chin. J. Catal. 2017, 38, 2132–2140.
- Lu, H.; Yan, Y.; Zhang, M.; Tan, H.; Geng, P.; Le, S.; Yang, Z.; Liu, Y. The effects of adjusting pulse anodization parameters on the surface morphology and properties of a WO3 photoanode for photoelectrochemical water splitting. J. Solid State Electrochem. 2018, 22, 2169–2181.
- Lv, X.; Rodriguez, I.; Hu, C.; Shang, J.; Sit, P.H.L.; Ye, C.; Oskam, G.; Teoh, W.Y. Modulated anodization synthesis of Sn-doped iron oxide with enhanced solar water splitting performance. Mater. Today Chem. 2019, 12, 7–15.
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O Nanowire Photocathodes for Efficient and Durable Solar Water Splitting. Nano Lett. 2016, 16, 1848–1857.
- Shi, W.; Zhang, X.; Li, S.; Zhang, B.; Wang, M.; Shen, Y. Carbon coated Cu2O nanowires for photo-electrochemical water splitting with enhanced activity. Appl. Surf. Sci. 2015, 358, 404–411.
- Ho, W.-J.; Hsiao, K.-Y.; Hu, C.-H.; Chuang, T.-W.; Liu, J.-J.; Chen, Y.-H. Characterized plasmonic effects of various metallic nanoparticles on silicon solar cells using the same anodic aluminum oxide mask for film deposition. Thin Solid Films 2017, 631, 64–71.
- Kwon, H.-C.; Kim, A.; Lee, H.; Lee, D.; Jeong, S.; Moon, J. Parallelized Nanopillar Perovskites for Semitransparent Solar Cells Using an Anodized Aluminum Oxide Scaffold. Adv. Energy Mater. 2016, 6, 1601055.
- Rahman, M.A.; Wong, Y.C.; Song, G.; Zhu, D.M.; Wen, C. Improvement on electrochemical performances of nanoporous titania as anode of lithium-ion batteries through annealing of pure titanium foils. J. Energy Chem. 2018, 27, 250–263.
- Ni, J.; Fu, S.; Wu, C.; Maier, J.; Yu, Y.; Li, L. Self-Supported Nanotube Arrays of Sulfur-Doped TiO2 Enabling Ultrastable and Robust Sodium Storage. Adv. Mater. 2016, 28, 2259–2265.
- Haskul, M.; Ülgen, A.T.; Döner, A. Fabrication and characterization of Ni modified TiO2 electrode as anode material for direct methanol fuel cell. Int. J. Hydrogen Energy 2020, 45, 4860–4874.
- Manikandan, M.; Vedarajan, R.; Kodiyath, R.; Abe, H.; Ueda, S.; Dakshnamoorthy, A.; Rajalakshmi, N.; Dhathathreyan, K.S.; Ramesh, G.V. Pt Decorated Free-Standing TiO2 Nanotube Arrays: Highly Active and Durable Electrocatalyst for Oxygen Reduction and Methanol Oxidation Reactions. J. Nanosci. Nanotechnol. 2016, 16, 8269–8278.
- Ahmed, F.; Pervez, S.A.; Aljaafari, A.; Alshoaibi, A.; Abuhimd, H.; Oh, J.; Koo, B.H. Fabrication of TiO2-Nanotube-Array-Based Supercapacitors. Micromachines 2019, 10, 742.
- Appadurai, T.; Subramaniyam, C.H.; Kuppusamy, R.; Karazhanov, S.; Subramanian, B. Electrochemical Performance of Nitrogen-Doped TiO2 Nanotubes as Electrode Material for Supercapacitor and Li-Ion Battery. Molecules 2019, 24, 2952.
- Cheng, G.; Bai, Q.; Si, C.; Yang, W.; Dong, C.; Wang, H.; Gao, Y.L.; Zhang, Z. Nickel oxide nanopetal-decorated 3D nickel network with enhanced pseudocapacitive properties. RSC Adv. 2015, 5, 15042–15051.
- Stepniowski, W.J.; Misiolek, W.Z. Review of Fabrication Methods, Physical Properties, and Applications of Nanostructured Copper Oxides Formed via Electrochemical Oxidation. Nanomaterials 2018, 8, 379.
- Losic, D.; Santos, A. Nanoporous Alumina Fabrication, Structure, Properties and Applications; Springer: London, UK, 2015; Volume 219.
- Macak, J.M.; Schmuki, P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim. Acta 2006, 52, 1258–1264.
- Sulka, G.D. Nanostructured Anodic Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2020.
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406.
- Buyukaksoy, A.; Fürstenhaupt, T.; Birss, V.I. First-time electrical characterization of nanotubular ZrO2 films for micro-solid oxide fuel cell applications. Nanoscale 2015, 7, 8428–8437.
- Zhang, J.; Li, Y.; Zhang, Y.; Qian, X.; Niu, R.; Hu, R.; Zhu, X.; Wang, X.; Zhu, J. The enhanced adhesion between overlong TiNxOy/MnO2 nanoarrays and Ti substrate: Towards flexible supercapacitors with high energy density and long service life. Nano Energy 2018, 43, 91–102.
- Cheong, Y.L.; Beh, K.P.; Yam, F.K.; Hassan, Z. Performance evaluation of titanium dioxide based dye-sensitized solar cells under the influence of anodization steps, nanotube length and ionic liquid-free redox electrolyte solvents. Superlattices Microstruct. 2016, 94, 74–84.
- Gao, Y.; Lin, Y.; Peng, Z.; Zhou, Q.; Fan, Z. Accelerating ion diffusion with unique three-dimensionally interconnected nanopores for self-membrane high-performance pseudocapacitors. Nanoscale 2017, 9, 18311–18317.
- Kang, J.S.; Kim, J.; Lee, M.J.; Son, Y.J.; Jeong, J.; Chung, D.Y.; Lim, A.; Choe, H.; Park, H.S.; Sung, Y.-E. Electrochemical synthesis of nanoporous tungsten carbide and its application as electrocatalysts for photoelectrochemical cells. Nanoscale 2017, 9, 5413–5424.
- Krumpmann, A.; Dervaux, J.; Derue, L.; Douhéret, O.; Lazzaroni, R.; Snyders, R.; Decroly, A. Influence of a sputtered compact TiO2 layer on the properties of TiO2 nanotube photoanodes for solid-state DSSCs. Mater. Des. 2017, 120, 298–306.
- Hossain, M.A.; Oh, S.; Lim, S. Fabrication of dye-sensitized solar cells using a both-ends-opened TiO2 nanotube/nanoparticle hetero-nanostructure. J. Ind. Eng. Chem. 2017, 51, 122–128.
- Saboo, T.; Tavella, F.; Ampelli, C.; Perathoner, S.; Genovese, C.; Marepally, B.C.; Veyre, L.; Quadrelli, E.A.; Centi, G. Water splitting on 3D-type meso/macro porous structured photoanodes based on Ti mesh. Sol. Energy Mater. Sol. Cells 2018, 178, 98–105.
- Abraham, B.G.; Maniam, K.K.; Kuniyil, A.; Chetty, R. Electrocatalytic Performance of Palladium Dendrites Deposited on Titania Nanotubes for Formic Acid Oxidation. Fuel Cells 2016, 16, 656–661.
- Zhang, M.; Wang, C.; Li, H.; Wang, J.; Li, M.; Chen, X. Enhanced performance of lithium ion batteries from self-doped TiO2 nanotube anodes via an adjustable electrochemical process. Electrochim. Acta 2019, 326, 134972.
- Salian, G.D.; Koo, B.M.; Lefevre, C.; Cottineau, T.; Lebouin, C.; Tesfaye, A.T.; Knauth, P.; Keller, V.; Djenizian, T. Niobium Alloying of Self-Organized TiO2 Nanotubes as an Anode for Lithium-Ion Microbatteries. Adv. Mater. Technol. 2018, 3, 1700274.
- Pisarek, M.; Kędzierzawski, P.; Andrzejczuk, M.; Hołdyński, M.; Mikołajczuk-Zychora, A.; Borodziński, A.; Janik-Czachor, M. TiO2 Nanotubes with Pt and Pd Nanoparticles as Catalysts for Electro-Oxidation of Formic Acid. Materials 2020, 13, 1195.
- Madian, M.; Ummethala, R.; El Naga, A.O.A.; Ismail, N.; Rümmeli, M.H.; Eychmüller, A.; Giebele, L. Ternary CNTs@TiO2/CoO Nanotube Composites: Improved Anode Materials for High Performance Lithium Ion Batteries. Materials 2017, 10, 678.
- Guo, T.; Wang, C.; Xu, P.; Feng, C.; Si, S.; Zhang, Y.; Wang, Q.; Shi, M.; Yang, F.; Wang, J.; et al. Effects of the Structure of TiO2 Nanotube Arrays on Its Catalytic Activity for Microbial Fuel Cell. Glob. Chall. 2019, 3, 1800084.
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the Photonic Crystal Properties of TiO2 Nanotube Arrays To Enhance Photocatalytic Hydrogen Production. ACS Catal. 2016, 6, 1345–1353.
- Huang, L.; Zhang, X.; Shen, D.; Li, N.; Ge, Z.; Zhou, Y.; Zhou, M.; Feng, H.; Guo, K. Effect of heat-treatment atmosphere on the current generation of TiO2 nanotube array electrodes in microbial fuel cells. Electrochim. Acta 2017, 257, 203–209.
